Acids And Bases In Our Homes: A Chemical Journey Through Everyday Items
Acids and Bases in Our Homes: A Chemical Journey Through Everyday Items
Related Articles: Acids and Bases in Our Homes: A Chemical Journey Through Everyday Items
Introduction
With great pleasure, we will explore the intriguing topic related to Acids and Bases in Our Homes: A Chemical Journey Through Everyday Items. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Acids and Bases in Our Homes: A Chemical Journey Through Everyday Items

The world around us, including our homes, is teeming with chemical compounds, many of which are classified as acids or bases. These substances, often encountered in everyday items, play crucial roles in various household activities, from cleaning and cooking to personal care and even maintaining our health. Understanding the properties and applications of acids and bases provides valuable insights into the chemical nature of our surroundings and empowers us to make informed decisions about their use.
Defining Acids and Bases: A Foundation for Understanding
Before delving into the specific examples of acids and bases in household items, it is essential to establish a clear understanding of these chemical concepts. Acids and bases are defined by their distinct chemical properties and the way they interact with each other.
Acids: Acids are substances that release hydrogen ions (H+) when dissolved in water. This release of hydrogen ions is what gives acids their characteristic sour taste and ability to react with bases. Some common examples of acids include:
- Hydrochloric acid (HCl): Found in stomach acid, hydrochloric acid aids in digestion by breaking down food.
- Acetic acid (CH3COOH): The primary component of vinegar, acetic acid is used in cooking, cleaning, and preserving food.
- Citric acid (C6H8O7): Found naturally in citrus fruits, citric acid adds a tangy flavor and is used as a preservative in food and beverages.
- Lactic acid (C3H6O3): Produced by bacteria during the fermentation of milk, lactic acid gives yogurt its characteristic tart flavor and is also used in skincare products.
Bases: Bases, also known as alkalis, are substances that release hydroxide ions (OH-) when dissolved in water. They have a bitter taste and feel slippery to the touch. Some common examples of bases include:
- Sodium hydroxide (NaOH): Also known as lye, sodium hydroxide is a strong base used in soap making, drain cleaning, and paper production.
- Potassium hydroxide (KOH): Similar to sodium hydroxide, potassium hydroxide is used in various industrial applications, including soap making and battery production.
- Ammonia (NH3): A common household cleaner, ammonia is a weak base used to disinfect surfaces and remove grease.
- Baking soda (NaHCO3): A versatile substance, baking soda is a weak base used in cooking, cleaning, and as an antacid.
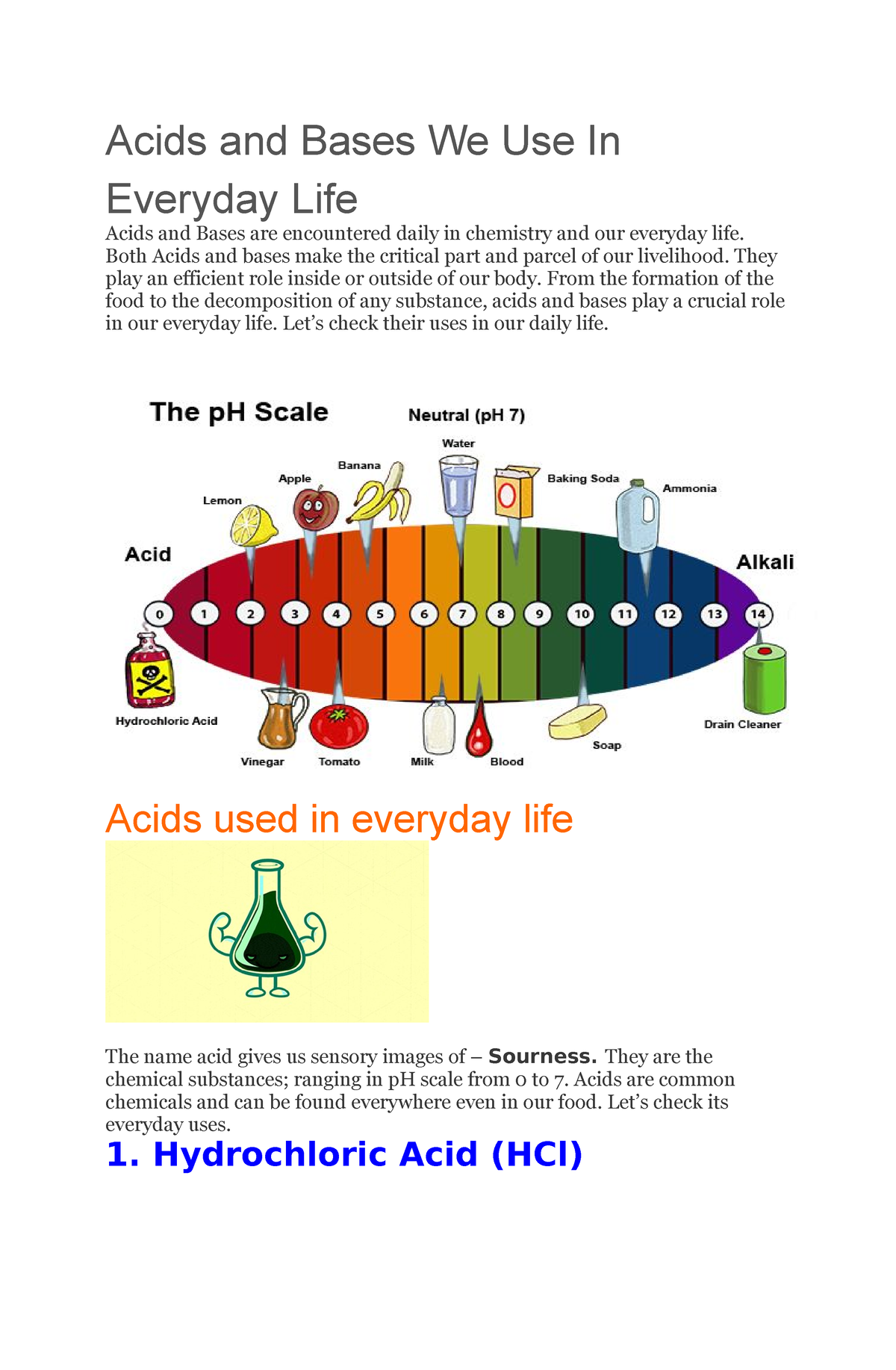
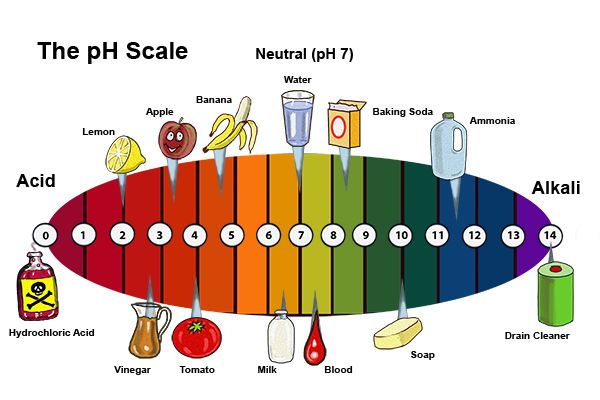
The pH Scale: Measuring Acidity and Alkalinity
The pH scale provides a quantitative measure of the acidity or alkalinity of a solution. It ranges from 0 to 14, with 7 being neutral. Solutions with a pH less than 7 are acidic, while solutions with a pH greater than 7 are basic or alkaline. The further a solution is from 7, the stronger its acidity or alkalinity.
Acids and Bases in Household Items: A Detailed Exploration
Now, let’s explore the specific examples of acids and bases commonly found in our homes and their diverse applications:
Cleaning Products:
- Vinegar: This acidic solution, primarily acetic acid, is a versatile cleaning agent. It can be used to clean surfaces, remove mineral deposits, deodorize, and even kill weeds.
- Lemon juice: Containing citric acid, lemon juice acts as a natural cleaning agent, effectively removing stains and odors.
- Baking soda: As a weak base, baking soda is an excellent abrasive cleaner. It can be used to scrub surfaces, remove stains, and deodorize.
- Ammonia: A strong base, ammonia is commonly used as a disinfectant and grease remover. It can be mixed with water to create a cleaning solution for various surfaces.
Cooking and Baking:
- Vinegar: In cooking, vinegar adds a tangy flavor and acts as a tenderizer for meats. It is also used in pickling and preserving food.
- Lemon juice: Similar to vinegar, lemon juice adds a tangy flavor and is used in marinades, sauces, and desserts.
- Baking soda: A key ingredient in baking, baking soda reacts with acids in recipes, producing carbon dioxide gas, which causes cakes and bread to rise.
- Baking powder: This leavening agent contains both baking soda and an acidic component, allowing it to react with moisture and produce carbon dioxide for baking.
Personal Care:
- Soap: Soap is made by reacting fats or oils with a strong base like sodium hydroxide or potassium hydroxide. The resulting soap molecules contain both hydrophobic (water-repelling) and hydrophilic (water-attracting) ends, allowing them to effectively remove dirt and grime.
- Shampoo: Some shampoos contain acidic ingredients like citric acid or lactic acid to help balance the pH of the scalp and hair.
- Toothpaste: Many toothpastes contain weak acids like phosphoric acid or citric acid to help remove plaque and whiten teeth.
Health and Wellness:
- Antacids: Antacids are often used to relieve heartburn and indigestion. They contain weak bases like calcium carbonate or magnesium hydroxide, which neutralize excess stomach acid.
- Vitamins: Some vitamins, like vitamin C (ascorbic acid), are acidic in nature. These vitamins play crucial roles in maintaining overall health.
- Electrolyte solutions: Electrolyte solutions, used to rehydrate the body after dehydration, often contain acids and bases to help restore the body’s electrolyte balance.
Other Applications:
- Battery acid: Car batteries contain sulfuric acid, a strong acid that serves as an electrolyte.
- Rust removal: Acids like citric acid or phosphoric acid can be used to remove rust from metal surfaces.
- Pool maintenance: Pool water is typically kept slightly basic (alkaline) to prevent corrosion and maintain the proper pH balance for swimming.
FAQs: Addressing Common Questions About Acids and Bases in Household Items
Q: Are all acids and bases dangerous?
A: Not all acids and bases are dangerous. Many are present in everyday items and are safe for use in appropriate concentrations. However, some acids and bases, especially strong ones, can be corrosive and harmful if not handled properly. It is crucial to always follow safety instructions and use protective gear when handling concentrated acids and bases.
Q: How can I safely handle acids and bases in my home?
A: Always wear gloves and eye protection when handling acids and bases. Avoid mixing different cleaning products, as this can create dangerous reactions. Store acids and bases in their original containers, away from heat and sunlight. Never pour water into concentrated acid, as this can cause a violent reaction.
Q: What happens when an acid and a base react?
A: When an acid and a base react, they neutralize each other, forming water and a salt. This reaction is called neutralization. For example, when hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH), it produces water (H2O) and sodium chloride (NaCl), which is table salt.
Q: How can I tell if a substance is acidic or basic?
A: You can use litmus paper to test the pH of a solution. Red litmus paper turns blue in basic solutions, while blue litmus paper turns red in acidic solutions.
Tips for Using Acids and Bases Safely in the Home:
- Read labels carefully: Always read the instructions and safety warnings on the labels of cleaning products and other household items containing acids or bases.
- Dilute concentrated solutions: If using concentrated acids or bases, always dilute them with water before using them.
- Use appropriate containers: Store acids and bases in their original containers, which are designed to be safe and prevent spills.
- Keep them out of reach of children: Always keep acids and bases out of reach of children and pets.
- Ventilate the area: When using acids or bases, ensure adequate ventilation to prevent the buildup of fumes.
- Use gloves and eye protection: Wear gloves and eye protection when handling acids and bases to protect your skin and eyes.
- Neutralize spills immediately: If an acid or base spills, neutralize it immediately with a suitable substance, like baking soda for acids or vinegar for bases.
Conclusion: Embracing the Chemistry of Our Homes
Acids and bases are integral components of our everyday lives, playing vital roles in cleaning, cooking, personal care, and even maintaining our health. Understanding their properties and applications allows us to make informed decisions about their use, ensuring safe and effective utilization in our homes. By embracing the chemistry of our surroundings, we can better appreciate the intricate interplay of molecules that shape our daily experiences.





/acid-and-base-combined-58dad2a63df78c5162f364e6.jpg)
Closure
Thus, we hope this article has provided valuable insights into Acids and Bases in Our Homes: A Chemical Journey Through Everyday Items. We hope you find this article informative and beneficial. See you in our next article!