Alpha Particles: Their Sources And Applications
Alpha Particles: Their Sources and Applications
Related Articles: Alpha Particles: Their Sources and Applications
Introduction
With enthusiasm, let’s navigate through the intriguing topic related to Alpha Particles: Their Sources and Applications. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
- 1 Related Articles: Alpha Particles: Their Sources and Applications
- 2 Introduction
- 3 Alpha Particles: Their Sources and Applications
- 3.1 Naturally Occurring Sources of Alpha Particles: The Radioactive Decay of Heavy Elements
- 3.2 Man-Made Sources of Alpha Particles: The Power of Nuclear Reactors
- 3.3 Applications of Alpha Particles: Harnessing Energy and Precision
- 3.4 FAQs about Alpha Particles: Addressing Common Concerns
- 3.5 Tips for Safe Handling of Alpha Emitters: Prioritizing Safety
- 3.6 Conclusion: Balancing Risks and Rewards
- 4 Closure
Alpha Particles: Their Sources and Applications
Alpha particles, consisting of two protons and two neutrons, are a type of ionizing radiation emitted by certain radioactive isotopes. Their relatively large size and positive charge make them highly energetic and capable of causing significant biological damage. While their potential hazards are well-documented, alpha particles also possess unique properties that make them valuable tools in various scientific and industrial applications.
Naturally Occurring Sources of Alpha Particles: The Radioactive Decay of Heavy Elements
The primary source of alpha particles in nature is the radioactive decay of heavy elements like uranium, thorium, and radium. These elements possess unstable nuclei that undergo alpha decay, releasing alpha particles as they transform into lighter, more stable elements. This process occurs spontaneously and continuously, contributing to the natural background radiation present in our environment.
Uranium: The most abundant naturally occurring source of alpha particles is uranium. Uranium-238, the most common isotope, decays through a series of alpha and beta decays, ultimately transforming into lead-206. This decay chain releases multiple alpha particles, contributing significantly to the alpha radiation found in rocks, soil, and water.
Thorium: Similar to uranium, thorium-232 is another naturally occurring radioactive element that undergoes alpha decay. Its decay chain, leading to lead-208, also releases multiple alpha particles. Thorium is found in various minerals and is a significant contributor to the natural alpha radiation background.
Radium: Radium, a radioactive element discovered in 1898, is known for its intense alpha particle emission. Radium-226, the most common isotope, decays through a series of alpha and beta decays, ultimately transforming into lead-206. Its high alpha activity made it historically used in luminous paints and medical treatments, but its hazardous nature has led to its largely discontinued use.
Man-Made Sources of Alpha Particles: The Power of Nuclear Reactors
Nuclear reactors, designed to harness the energy released during nuclear fission, are another significant source of alpha particles. The fission process involves the splitting of heavy atomic nuclei, such as uranium-235, into lighter nuclei, accompanied by the release of energy, neutrons, and alpha particles.
Fission Products: The fission process produces a wide range of radioactive isotopes, many of which emit alpha particles. These fission products, including isotopes of plutonium, americium, and curium, are highly radioactive and pose significant health risks.
Neutron Activation: Neutrons released during fission can interact with other elements present in the reactor core, leading to neutron activation. This process can create new radioactive isotopes, some of which also emit alpha particles. For instance, neutron activation of cobalt-59 produces cobalt-60, a significant source of gamma rays and, to a lesser extent, alpha particles.
Applications of Alpha Particles: Harnessing Energy and Precision
Despite their potential hazards, alpha particles possess unique properties that make them valuable tools in various fields:
Alpha Particle Spectroscopy: Alpha particles emitted by radioactive isotopes have distinct energies, allowing them to be used in alpha particle spectroscopy. This technique analyzes the energy spectrum of emitted alpha particles to identify the specific radioactive isotopes present in a sample. Applications range from geological dating to nuclear forensics and environmental monitoring.
Smoke Detectors: Americium-241, a man-made alpha emitter, is commonly used in smoke detectors. The alpha particles emitted by americium-241 ionize air molecules, creating a small electrical current. When smoke particles enter the detector, they disrupt this current, triggering an alarm.
Radiotherapy: Alpha particle therapy, a relatively new cancer treatment modality, utilizes the high energy and short range of alpha particles to target and destroy cancerous cells with minimal damage to surrounding healthy tissue. Alpha particles are particularly effective in treating superficial cancers and are being investigated for their potential in treating other types of cancer.
Space Exploration: Alpha particles are used in instruments aboard spacecraft to study the composition and properties of planetary surfaces and atmospheres. For example, alpha particle spectrometers are used to analyze the elemental composition of rocks and soils on Mars, providing valuable insights into its geological history.
Industrial Applications: Alpha particles are used in various industrial applications, such as gauging the thickness of materials, measuring the density of liquids, and detecting the presence of contaminants. Their ability to ionize matter makes them suitable for these applications, where they can interact with materials and provide valuable information.
FAQs about Alpha Particles: Addressing Common Concerns
Q: Are alpha particles dangerous?
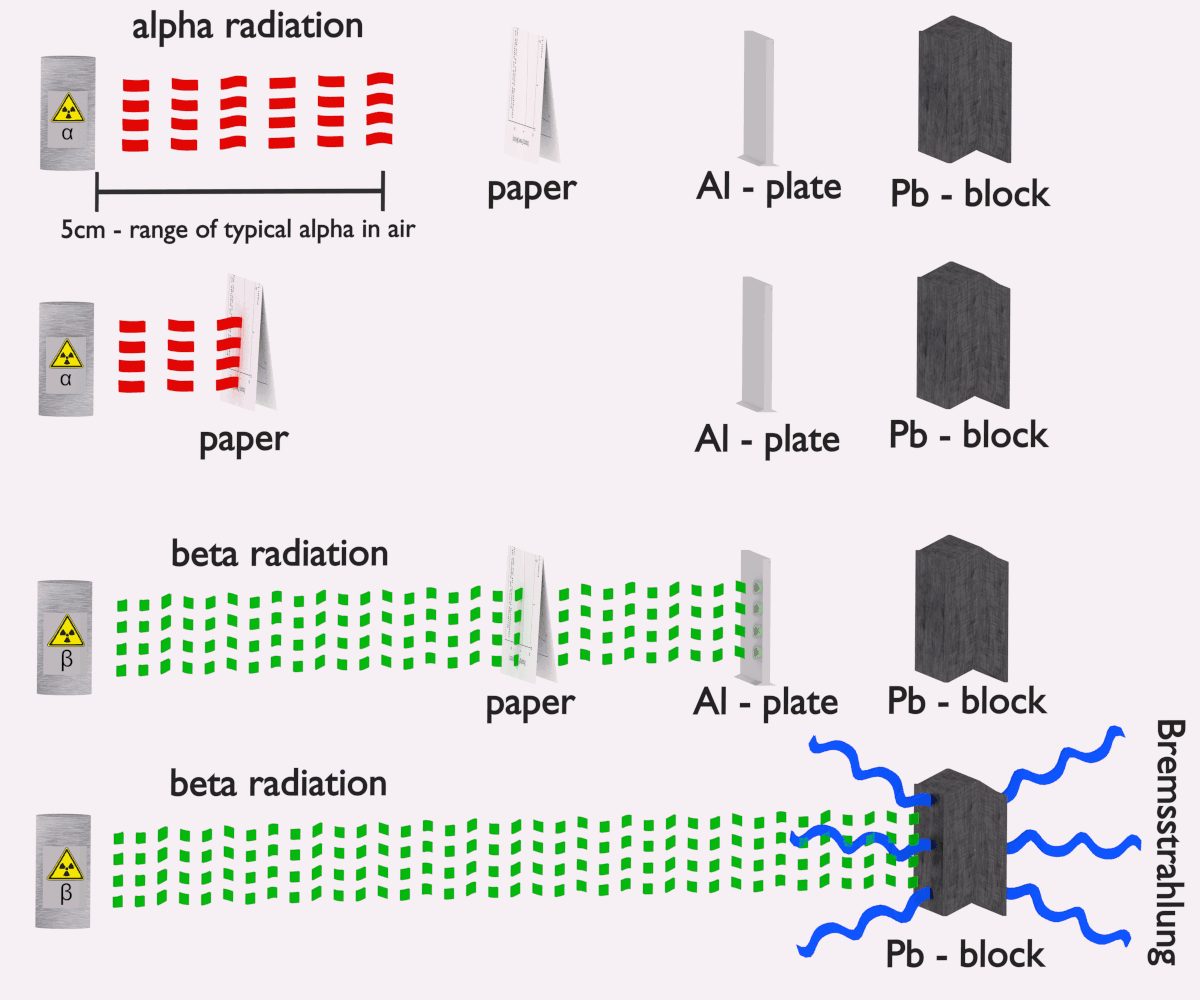
A: Yes, alpha particles are highly ionizing and can cause significant biological damage if they enter the body. However, their large size and positive charge mean they have a short range in air and are easily stopped by a sheet of paper or the outer layer of skin. The primary danger arises from ingestion or inhalation of alpha-emitting materials, allowing them to directly damage internal tissues.
Q: How can I protect myself from alpha particles?
A: The best way to protect yourself from alpha particles is to avoid exposure to alpha-emitting materials. This involves handling radioactive materials with appropriate safety precautions, such as wearing protective clothing and using shielded containers. It’s also important to be aware of the natural background radiation levels in your environment and take steps to minimize exposure where possible.
Q: Are alpha particles used in nuclear weapons?
A: While alpha particles are released during nuclear fission, they are not the primary source of energy in nuclear weapons. The primary energy release comes from the fission process itself, which generates a large amount of heat and radiation. However, alpha particles can contribute to the overall radioactive fallout from a nuclear explosion.
Q: How can I tell if something is emitting alpha particles?
A: It’s generally not possible to detect alpha particles with the naked eye. Specialized instruments like Geiger counters and scintillation detectors are required to measure alpha radiation.
Tips for Safe Handling of Alpha Emitters: Prioritizing Safety
- Always wear appropriate personal protective equipment (PPE), such as gloves, lab coats, and respirators, when handling alpha-emitting materials.
- Work in a well-ventilated area to minimize the risk of inhaling alpha particles.
- Use shielded containers to store and transport alpha-emitting materials.
- Follow all safety protocols and procedures established by your institution or regulatory body.
- Be aware of the potential hazards associated with alpha particles and take appropriate precautions.
Conclusion: Balancing Risks and Rewards
Alpha particles are a fascinating and powerful form of ionizing radiation. While their potential hazards are undeniable, their unique properties make them valuable tools in various scientific and industrial applications. By understanding the sources, properties, and applications of alpha particles, we can harness their potential while mitigating the associated risks, contributing to advancements in diverse fields, from medicine to space exploration.


.PNG)


Closure
Thus, we hope this article has provided valuable insights into Alpha Particles: Their Sources and Applications. We appreciate your attention to our article. See you in our next article!
