The Chemistry Of Our Kitchens: Exploring Acids And Bases In Everyday Life
The Chemistry of Our Kitchens: Exploring Acids and Bases in Everyday Life
Related Articles: The Chemistry of Our Kitchens: Exploring Acids and Bases in Everyday Life
Introduction
With great pleasure, we will explore the intriguing topic related to The Chemistry of Our Kitchens: Exploring Acids and Bases in Everyday Life. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
The Chemistry of Our Kitchens: Exploring Acids and Bases in Everyday Life

The world around us is a tapestry woven with chemical reactions, and our homes are no exception. Within the seemingly mundane objects of our daily lives, we encounter a fascinating interplay of acids and bases. These chemical entities, often invisible to the naked eye, play vital roles in various household tasks, from cleaning and cooking to personal care and maintaining a healthy environment.
This article delves into the realm of common household acids and bases, revealing their properties, applications, and the crucial role they play in our everyday lives.
Understanding Acids and Bases: A Primer
Before embarking on our exploration, it is essential to grasp the fundamental concepts of acids and bases.
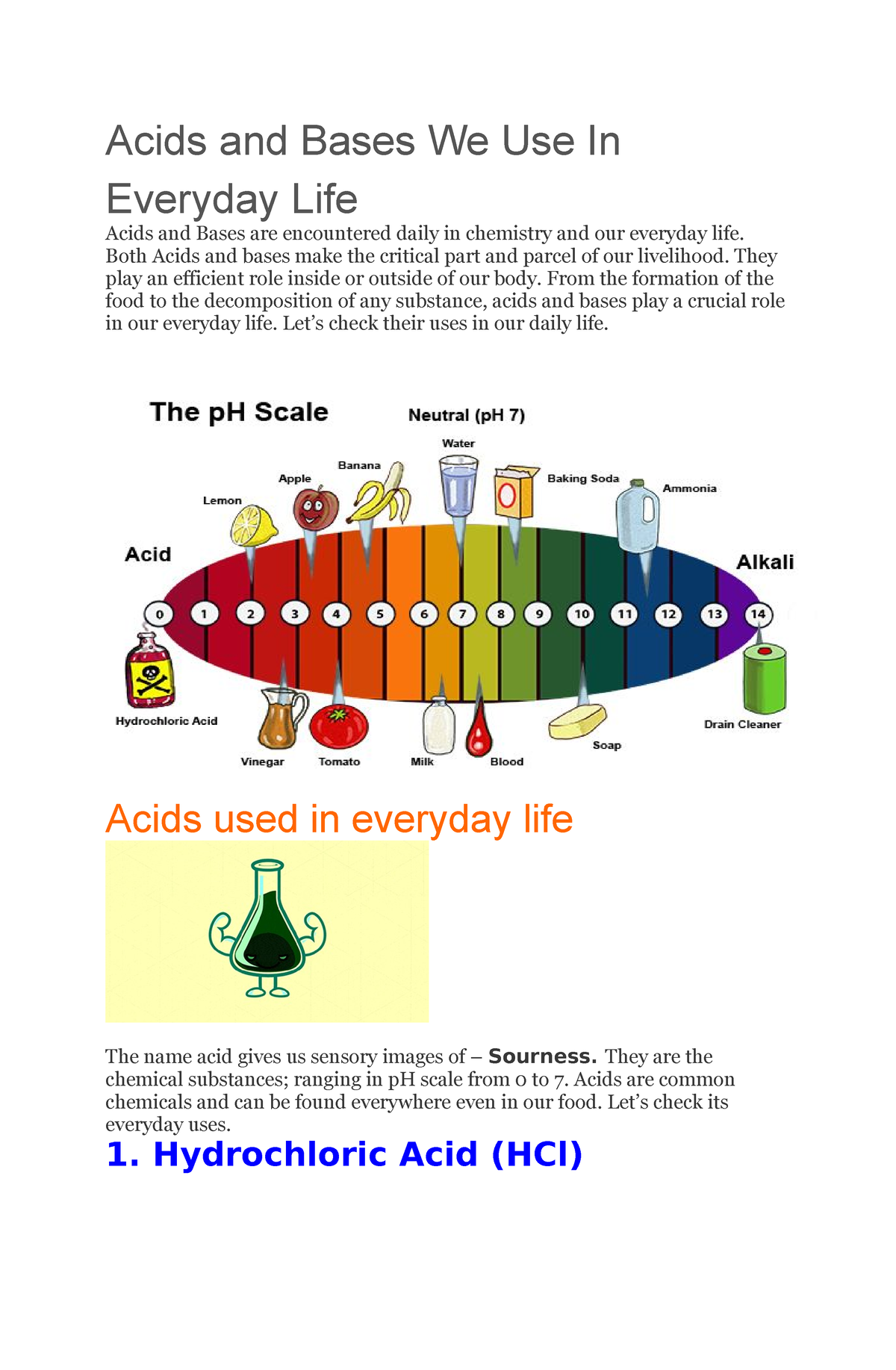
Acids are substances that release hydrogen ions (H+) when dissolved in water. They typically possess a sour taste, can react with metals to produce hydrogen gas, and turn blue litmus paper red. Examples of common acids include lemon juice, vinegar, and stomach acid.
Bases, also known as alkalis, are substances that release hydroxide ions (OH-) when dissolved in water. They are typically characterized by a bitter taste, a slippery feel, and the ability to turn red litmus paper blue. Common examples include baking soda, soap, and ammonia.
The strength of an acid or base is determined by its ability to donate or accept hydrogen ions, respectively. Strong acids and bases ionize completely in solution, while weak acids and bases only partially ionize.
Common Household Acids
-
Vinegar: A staple in most kitchens, vinegar is a dilute solution of acetic acid. Its acidic nature makes it a versatile cleaning agent, capable of removing mineral deposits, dissolving grease, and even acting as a natural weed killer.
-
Lemon Juice: Rich in citric acid, lemon juice is a natural disinfectant and a popular ingredient in various culinary creations. Its acidic properties can help brighten teeth, remove stains from fabrics, and even add a tangy flavor to dishes.
-
Carbonated Drinks: These beverages contain carbonic acid, formed by the dissolution of carbon dioxide in water. The fizz and tartness of carbonated drinks are due to the presence of this weak acid.
-
Battery Acid: Found in car batteries, sulfuric acid is a strong acid that plays a crucial role in the electrochemical reactions that power the vehicle. It is highly corrosive and must be handled with extreme caution.
-
Aspirin: A common pain reliever, aspirin (acetylsalicylic acid) is a weak acid that inhibits the production of prostaglandins, chemicals responsible for pain and inflammation.
Common Household Bases
-
Baking Soda: Sodium bicarbonate, commonly known as baking soda, is a mild base used extensively in baking, cleaning, and even as an antacid. Its ability to neutralize acids makes it an effective cleaning agent for removing grease and odors.
-
Soap: Soaps are made by reacting fats or oils with a strong base, typically sodium hydroxide (lye). The resulting soap molecules have a polar head that attracts water and a non-polar tail that attracts grease, allowing for the removal of dirt and grime.
-
Ammonia: A colorless gas with a pungent odor, ammonia is a strong base commonly used as a cleaning agent. Its alkaline properties make it effective at dissolving grease, cleaning windows, and removing stains.
-
Bleach: Sodium hypochlorite, the active ingredient in bleach, is a strong base that acts as a powerful disinfectant and bleaching agent. It is used to kill bacteria, remove stains, and brighten fabrics.
-
Milk of Magnesia: Magnesium hydroxide, commonly known as milk of magnesia, is a mild base used as an antacid to neutralize excess stomach acid. It is also used as a laxative to relieve constipation.
The Importance of Acids and Bases in Our Lives
Acids and bases are not merely chemical curiosities; they play a vital role in maintaining life and ensuring the smooth functioning of our world.
Biological Systems: Our bodies rely on the delicate balance of acids and bases to maintain proper pH levels. The stomach uses hydrochloric acid for digestion, while the blood relies on a buffer system to regulate its pH within a narrow range.
Industrial Processes: Acids and bases are essential components of numerous industrial processes. Acids are used in the production of fertilizers, plastics, and detergents, while bases are used in the manufacture of paper, soap, and textiles.
Environmental Protection: Acids and bases are used in water treatment plants to adjust pH levels and remove impurities. They are also used in the remediation of soil and water contaminated with heavy metals.
Safety Precautions and Handling
While acids and bases are ubiquitous in our lives, it is crucial to handle them with care and respect. Strong acids and bases can cause severe burns and damage to skin, eyes, and respiratory systems. Always wear appropriate protective gear, such as gloves, goggles, and a respirator, when handling these substances.
FAQs
Q: How can I tell if a substance is an acid or a base?
A: You can use litmus paper to determine the pH of a substance. Acids turn blue litmus paper red, while bases turn red litmus paper blue.
Q: What happens when an acid and a base react?
A: When an acid and a base react, they undergo a neutralization reaction. This reaction produces salt and water, and the pH of the solution becomes closer to neutral (pH 7).
Q: What are some common household uses of acids and bases?
A: Acids are used in cleaning, cooking, and personal care. Bases are used in cleaning, baking, and as antacids.
Q: Are all acids and bases harmful?
A: No, not all acids and bases are harmful. Many are essential for life and have beneficial applications. However, it is important to handle strong acids and bases with caution.
Tips for Safe Handling of Acids and Bases:
- Always wear appropriate protective gear when handling acids and bases.
- Store acids and bases separately to prevent accidental mixing.
- Never mix strong acids and bases unless under the guidance of a qualified professional.
- Dilute strong acids and bases with water carefully by adding acid or base to water, never the other way around.
- Keep acids and bases away from children and pets.
Conclusion
The world of acids and bases is a fascinating and often overlooked aspect of our everyday lives. From the tartness of lemon juice to the cleaning power of baking soda, these chemical entities play a vital role in our homes, our bodies, and the world around us. By understanding their properties, applications, and safety precautions, we can harness their power responsibly and appreciate their importance in our lives.





:max_bytes(150000):strip_icc()/acid-and-base-combined-58dad2a63df78c5162f364e6.jpg)
Closure
Thus, we hope this article has provided valuable insights into The Chemistry of Our Kitchens: Exploring Acids and Bases in Everyday Life. We thank you for taking the time to read this article. See you in our next article!