The PH Scale: A Guide To Acidity And Alkalinity In Everyday Life
The pH Scale: A Guide to Acidity and Alkalinity in Everyday Life
Related Articles: The pH Scale: A Guide to Acidity and Alkalinity in Everyday Life
Introduction
With enthusiasm, let’s navigate through the intriguing topic related to The pH Scale: A Guide to Acidity and Alkalinity in Everyday Life. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
The pH Scale: A Guide to Acidity and Alkalinity in Everyday Life

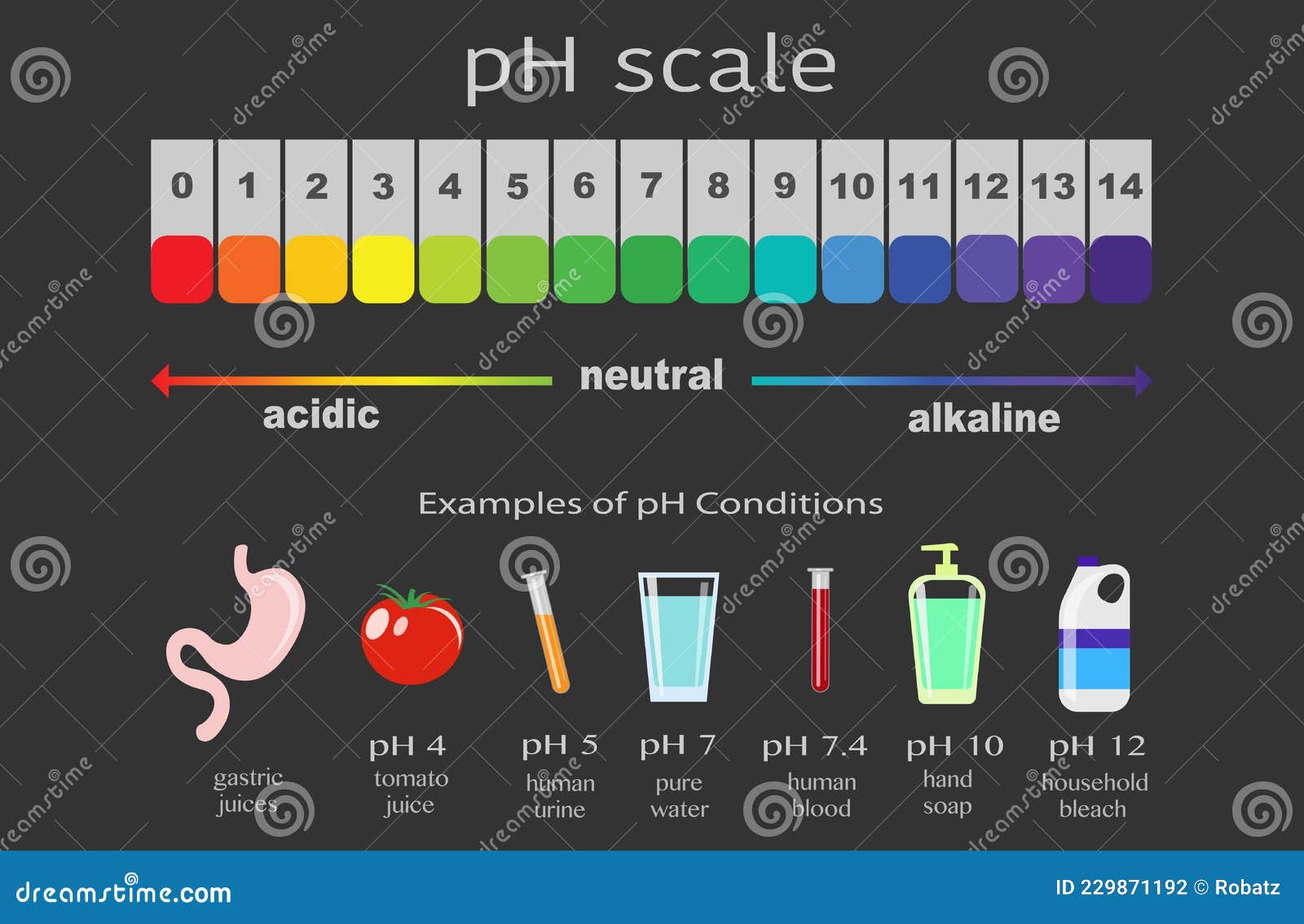
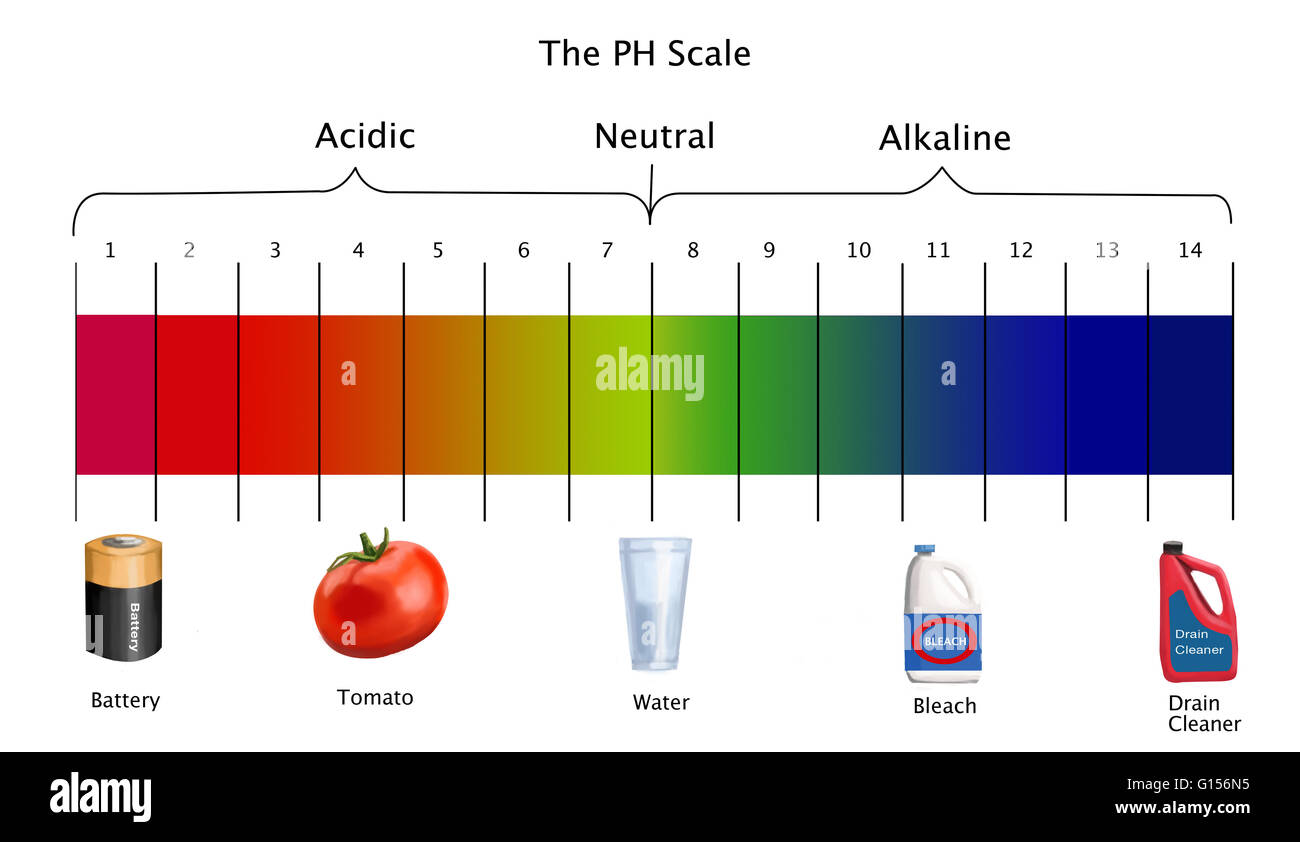
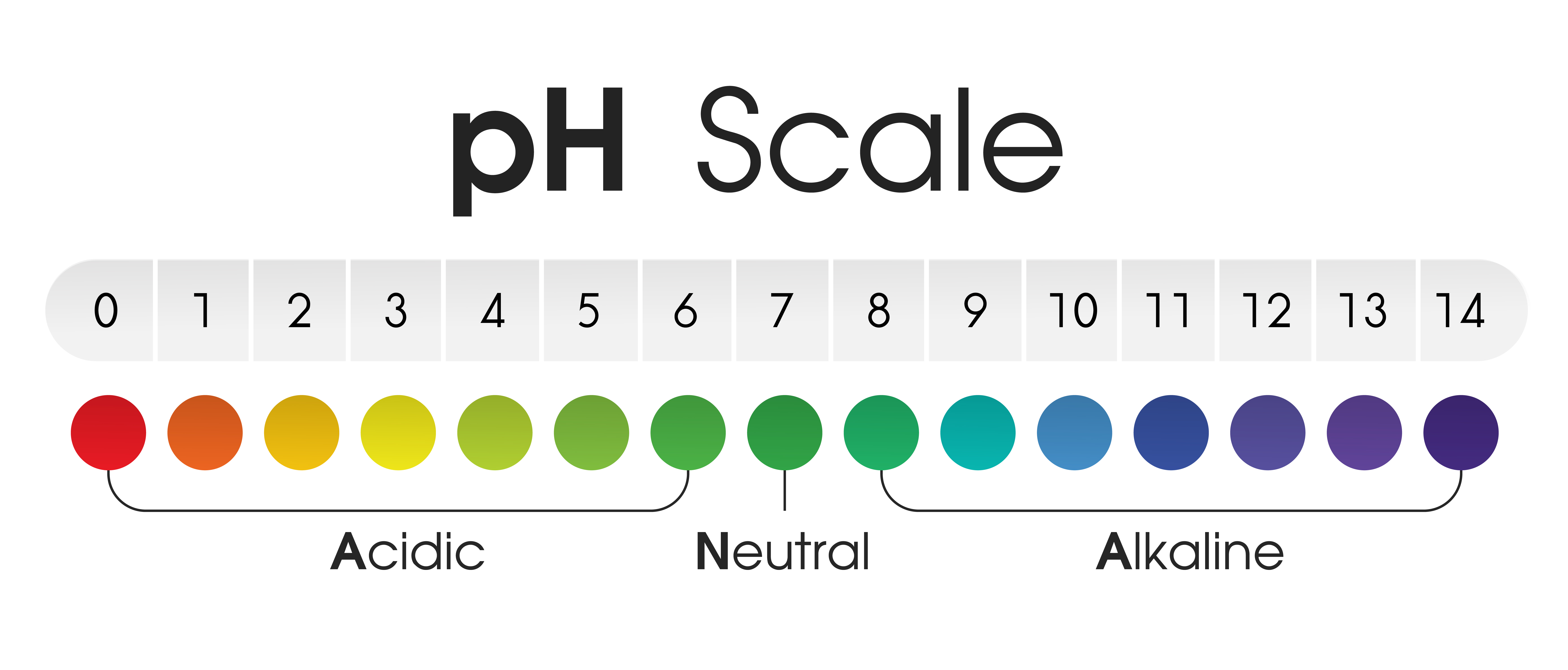
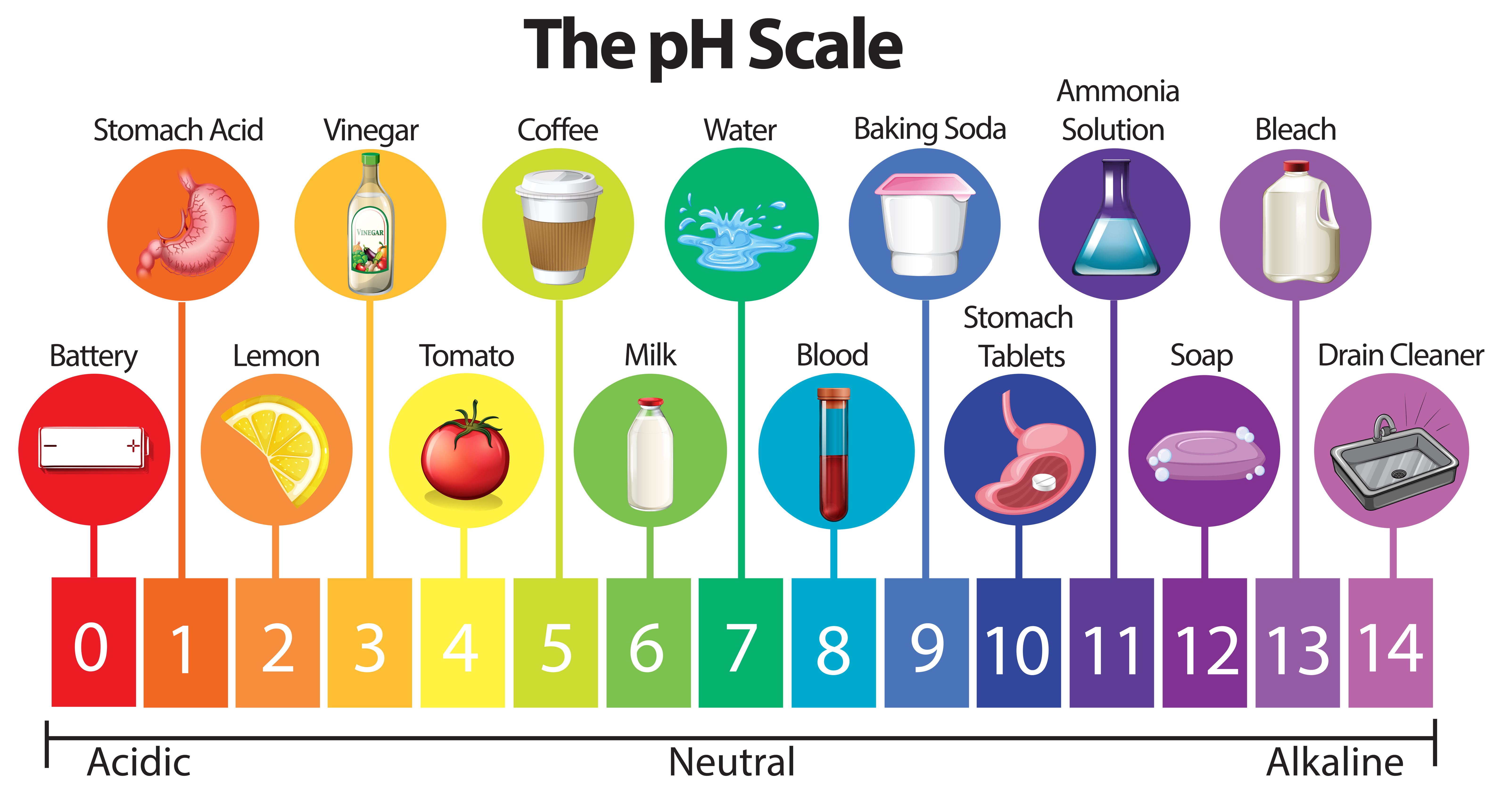
The pH scale is a numerical representation of the acidity or alkalinity of a solution. It ranges from 0 to 14, with 7 being neutral. Solutions with a pH below 7 are acidic, while those above 7 are alkaline (also known as basic). This seemingly simple scale has profound implications for various aspects of our lives, influencing everything from the food we eat to the health of our environment.
Understanding the pH Scale
The pH scale is based on the concentration of hydrogen ions (H+) in a solution. The more hydrogen ions present, the more acidic the solution. Conversely, a lower concentration of hydrogen ions indicates a more alkaline solution. The scale is logarithmic, meaning each whole number change in pH represents a tenfold change in the concentration of hydrogen ions.
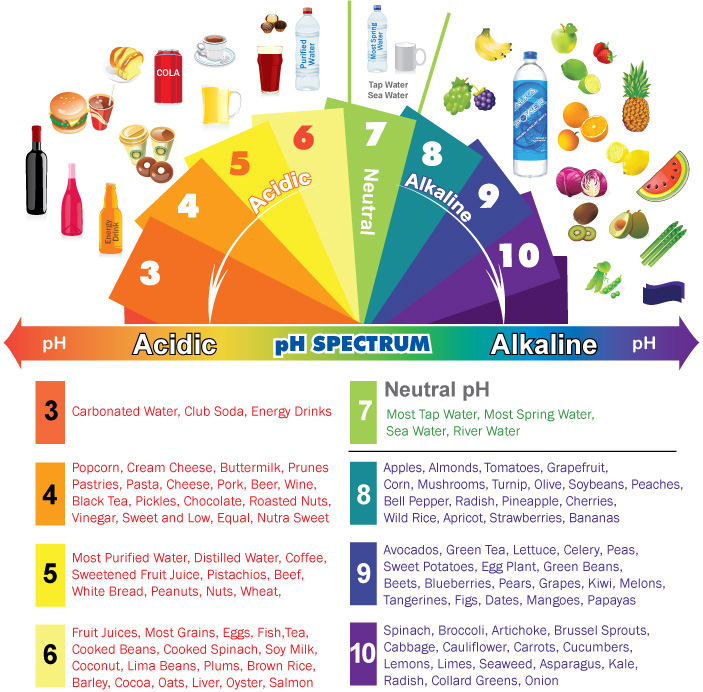
Common Items on the pH Scale
The pH scale encompasses a wide range of substances, from the highly acidic to the strongly alkaline. Understanding the pH of common items can provide valuable insights into their properties and uses.
Acids (pH below 7):
- Stomach Acid (pH 1.5-3.5): This highly acidic environment is essential for digestion, breaking down food and activating digestive enzymes.
- Lemon Juice (pH 2.0-2.4): The citric acid in lemons gives them their sour taste and is often used as a natural flavoring agent and cleaning solution.
- Vinegar (pH 2.4-3.4): Acetic acid, the primary component of vinegar, provides its tangy flavor and is used in cooking, cleaning, and preservation.
- Black Coffee (pH 4.5-5.5): The acidity of coffee contributes to its characteristic flavor and aroma.
- Tomatoes (pH 4.0-4.5): Despite their acidic nature, tomatoes are a versatile ingredient in many cuisines.
- Rainwater (pH 5.0-5.5): Pure rainwater is slightly acidic due to the presence of dissolved carbon dioxide, which forms carbonic acid.
Neutral (pH 7):
- Pure Water (pH 7): Distilled water, free from impurities, has a neutral pH.
- Human Blood (pH 7.35-7.45): Maintaining a stable blood pH within this narrow range is crucial for human health.
Alkaline (pH above 7):
- Baking Soda (pH 8.3-9.0): Sodium bicarbonate, commonly known as baking soda, is a mild alkali used in baking, cleaning, and as an antacid.
- Seawater (pH 8.1): The ocean’s salinity contributes to its slightly alkaline pH.
- Milk of Magnesia (pH 10.5): Magnesium hydroxide, a common antacid, has a high pH and is used to neutralize stomach acid.
- Ammonia (pH 11.5): A strong alkali used in cleaning products and as a fertilizer.
- Lye (pH 13.5): Sodium hydroxide, also known as lye, is a highly corrosive alkali used in soap making and drain cleaning.
The Importance of pH
The pH of various substances plays a crucial role in numerous aspects of our lives:
- Food and Beverage Industry: pH control is essential in food production, ensuring proper fermentation, preservation, and quality. For example, the pH of milk influences its shelf life and susceptibility to spoilage.
- Agriculture: Soil pH affects the availability of nutrients to plants. Maintaining an optimal pH range is crucial for healthy plant growth and crop yields.
- Water Treatment: pH adjustment is a critical step in water treatment processes, ensuring safe drinking water and preventing corrosion in pipes.
- Human Health: Maintaining the pH balance of bodily fluids, such as blood and urine, is crucial for overall health.
- Environmental Protection: The pH of water bodies can impact aquatic life and ecosystem health. Acid rain, for instance, can lower the pH of lakes and rivers, harming fish and other organisms.
FAQs by Common Items on the pH Scale
1. Why is stomach acid so acidic?
Stomach acid’s high acidity is essential for digestion. It breaks down food, particularly proteins, into smaller molecules that can be absorbed by the body. This acidic environment also activates digestive enzymes and kills harmful bacteria ingested with food.
2. What are the benefits of drinking lemon water?
Lemon water is a popular beverage, often touted for its health benefits. While lemons are acidic, they are rich in vitamin C and antioxidants. However, the diluted nature of lemon water does not significantly impact the pH of the body.
3. Can baking soda neutralize acid reflux?
Baking soda, a mild alkali, can temporarily relieve heartburn or acid reflux. However, its use should be limited and consulted with a healthcare professional as frequent use can lead to side effects.
4. How does soil pH affect plant growth?
Soil pH affects the availability of essential nutrients to plants. Each nutrient has an optimal pH range for uptake. For example, phosphorus is more available in slightly acidic soils, while calcium is more readily absorbed in slightly alkaline soils.
5. Why is it important to maintain a stable blood pH?
The pH of blood is tightly regulated within a narrow range (7.35-7.45) to ensure proper function of vital organs and enzymes. Deviations from this range can lead to serious health problems.
Tips by Common Items on the pH Scale
- Maintaining a healthy stomach: Consuming a balanced diet, avoiding excessive alcohol and caffeine, and managing stress can help maintain a healthy stomach pH.
- Optimizing soil pH: Regular soil testing and amendments, such as lime for acidic soils or sulfur for alkaline soils, can adjust soil pH for optimal plant growth.
- Safe handling of cleaning products: Always follow the manufacturer’s instructions when using cleaning products, especially those containing strong acids or alkalis.
- Understanding the pH of your body: Monitoring urine pH can provide insights into overall health and help identify potential imbalances.
Conclusion
The pH scale is a powerful tool for understanding the acidity and alkalinity of various substances and their impact on our lives. From the digestive processes in our bodies to the health of our environment, pH plays a vital role. By understanding the pH of common items and its implications, we can make informed choices about our diet, health, and the environment.



Closure
Thus, we hope this article has provided valuable insights into The pH Scale: A Guide to Acidity and Alkalinity in Everyday Life. We appreciate your attention to our article. See you in our next article!