Understanding Alpha Particles: Structure, Properties, And Applications
Understanding Alpha Particles: Structure, Properties, and Applications
Related Articles: Understanding Alpha Particles: Structure, Properties, and Applications
Introduction
With enthusiasm, let’s navigate through the intriguing topic related to Understanding Alpha Particles: Structure, Properties, and Applications. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Understanding Alpha Particles: Structure, Properties, and Applications

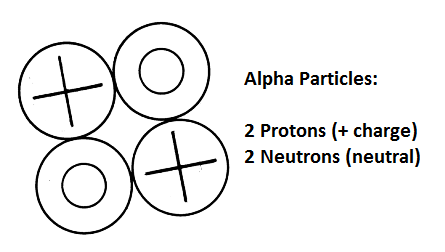
Alpha particles, a fundamental component of nuclear physics, play a crucial role in various scientific fields. These particles, consisting of two protons and two neutrons bound together, are essentially the nuclei of helium atoms. Their unique properties, including high energy and strong ionizing power, make them significant in areas such as nuclear decay, radiation therapy, and even the evolution of stars.
The Nature of Alpha Particles
Alpha particles are denoted by the symbol α and possess a +2 charge due to their two protons. Their mass is approximately four atomic mass units (amu), effectively the same as a helium nucleus. Their relatively large size and charge contribute to their notable characteristics:
- High Energy: Alpha particles are emitted with significant kinetic energy, typically in the range of several MeV (megaelectron volts). This energy stems from the nuclear reactions that produce them, such as alpha decay.
- Strong Ionizing Power: As they travel through matter, alpha particles interact strongly with atoms, stripping electrons from them and creating ions. This ionizing power is a consequence of their charge and relatively large size, leading to significant energy deposition along their paths.
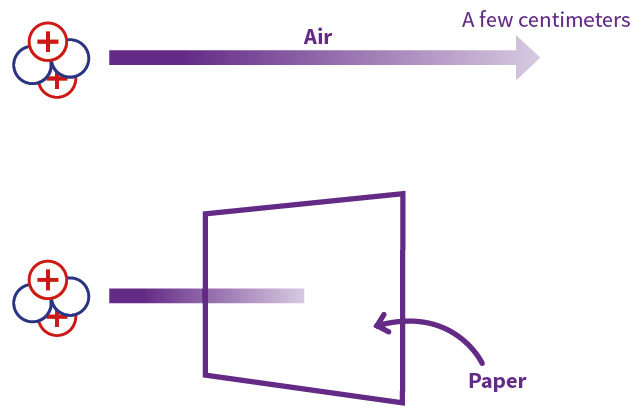
- Short Range: Despite their high energy, alpha particles have a limited range in matter. Their large size and charge cause them to lose energy rapidly through ionization, resulting in a short penetration depth. For example, a typical alpha particle emitted from a radioactive source can only travel a few centimeters in air.
Alpha Decay and the Role of Alpha Particles
Alpha decay is a radioactive process where an unstable atomic nucleus emits an alpha particle. This emission transforms the parent nucleus into a daughter nucleus with a lower atomic mass and atomic number. The process is governed by the principle of nuclear stability, where nuclei with an imbalance of protons and neutrons tend to decay to achieve a more stable configuration.
Alpha decay is a common phenomenon in heavy elements, such as uranium, thorium, and radium. These elements possess nuclei with a high number of protons, making them inherently unstable. By emitting alpha particles, they reduce their proton count, moving towards a more stable state.
Applications of Alpha Particles
The unique properties of alpha particles have led to their application in various fields, including:
- Radiation Therapy: Alpha particles, due to their high ionizing power and short range, are used in targeted radiation therapy for cancer treatment. They effectively kill cancerous cells while minimizing damage to surrounding healthy tissues.
- Smoke Detectors: Alpha particles are employed in ionization-type smoke detectors. The particles ionize the air in a chamber, creating a small electric current. When smoke enters the chamber, it absorbs the alpha particles, reducing the current and triggering an alarm.
- Nuclear Dating: Alpha decay, a well-defined process with a known decay rate, is used in radiometric dating techniques. By measuring the ratio of parent and daughter isotopes in a sample, scientists can determine its age.
- Astrophysics: Alpha particles are crucial in the nuclear fusion reactions that power stars. These reactions, occurring at extremely high temperatures and pressures, involve the fusion of hydrogen nuclei into helium nuclei, releasing vast amounts of energy.
FAQs Regarding Alpha Particles
Q: Are alpha particles dangerous?
A: Yes, alpha particles can be dangerous, especially if ingested or inhaled. Their high ionizing power can damage biological tissues. However, their short range means that external exposure to alpha particles is generally not a concern.
Q: How are alpha particles detected?
A: Alpha particles can be detected using various methods, including:
- Geiger-Müller counters: These detectors utilize the ionization effect of alpha particles to produce electrical pulses.
- Scintillation detectors: These detectors use materials that emit light when struck by alpha particles.
- Cloud chambers: These devices visualize the paths of alpha particles as they ionize the air, creating visible tracks.
Q: What is the difference between alpha particles and beta particles?
A: Alpha particles consist of two protons and two neutrons, while beta particles are either electrons or positrons. Beta particles have a much smaller mass and range than alpha particles.
Tips for Understanding Alpha Particles
- Visualize the structure: Imagine an alpha particle as a small, dense ball containing two protons and two neutrons.
- Connect the concepts: Understand the relationship between alpha decay, nuclear stability, and the emission of alpha particles.
- Focus on the applications: Explore the various ways alpha particles are used in different fields, such as medicine, technology, and astrophysics.
Conclusion
Alpha particles, with their unique properties of high energy, strong ionizing power, and short range, play a significant role in various scientific fields. From nuclear decay to radiation therapy and astrophysical processes, these particles offer valuable insights into the fundamental nature of matter and energy. Understanding their characteristics and applications provides a deeper appreciation for the complexities of the nuclear world.




Closure
Thus, we hope this article has provided valuable insights into Understanding Alpha Particles: Structure, Properties, and Applications. We hope you find this article informative and beneficial. See you in our next article!