Unveiling The Essence Of Alpha Particles: A Journey Into The Heart Of Radioactive Decay
Unveiling the Essence of Alpha Particles: A Journey into the Heart of Radioactive Decay
Related Articles: Unveiling the Essence of Alpha Particles: A Journey into the Heart of Radioactive Decay
Introduction
With enthusiasm, let’s navigate through the intriguing topic related to Unveiling the Essence of Alpha Particles: A Journey into the Heart of Radioactive Decay. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Unveiling the Essence of Alpha Particles: A Journey into the Heart of Radioactive Decay
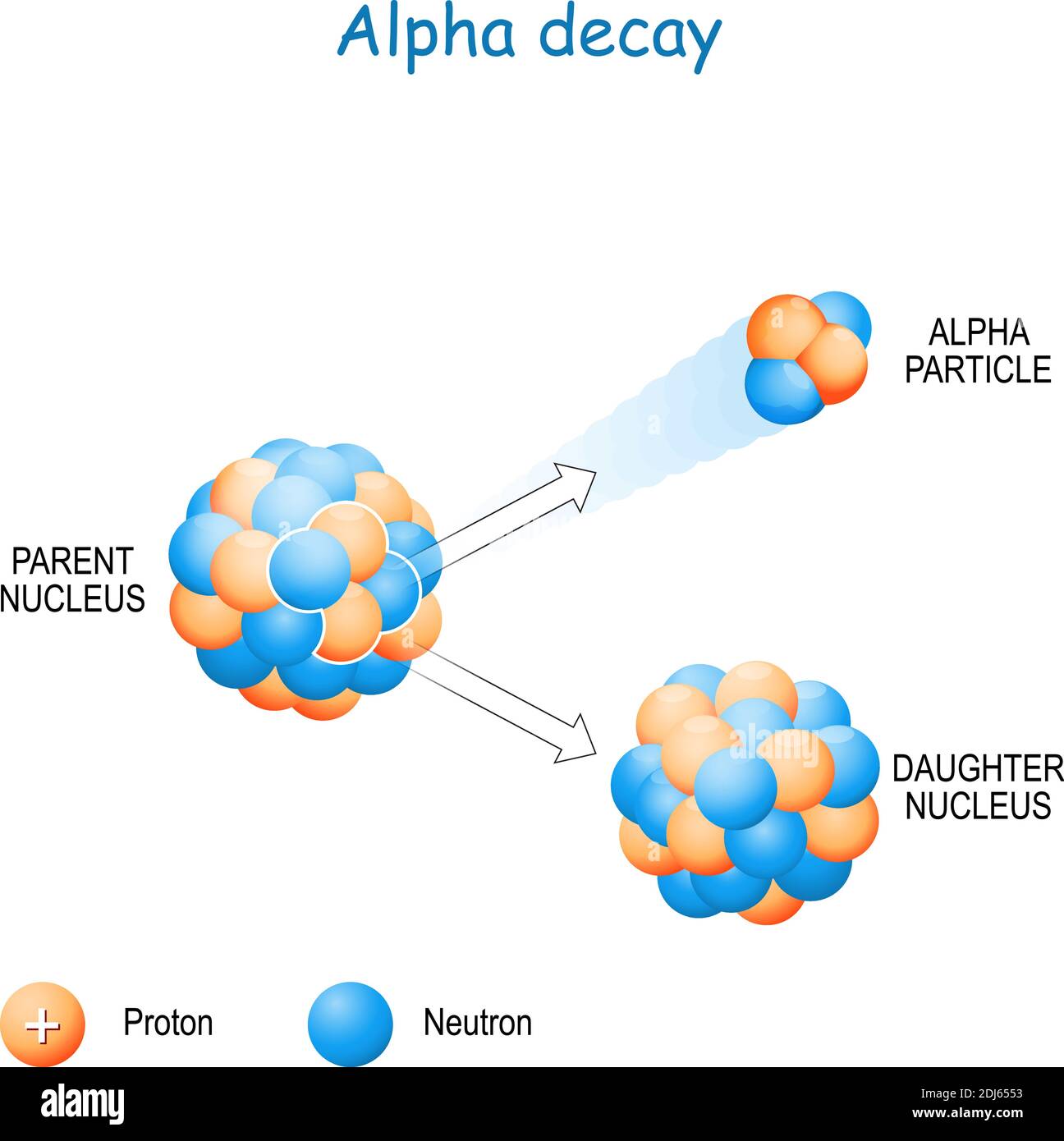
Alpha particles, a fundamental component of the radioactive decay process, hold a significant place in the realm of nuclear physics. Understanding their composition and behavior is crucial for comprehending the nature of radioactivity, its applications in various fields, and the potential risks associated with it. This article delves into the intricate world of alpha particles, exploring their structure, properties, and the implications of their emission.
The Core of Alpha Particles: Unveiling the Helium Nucleus
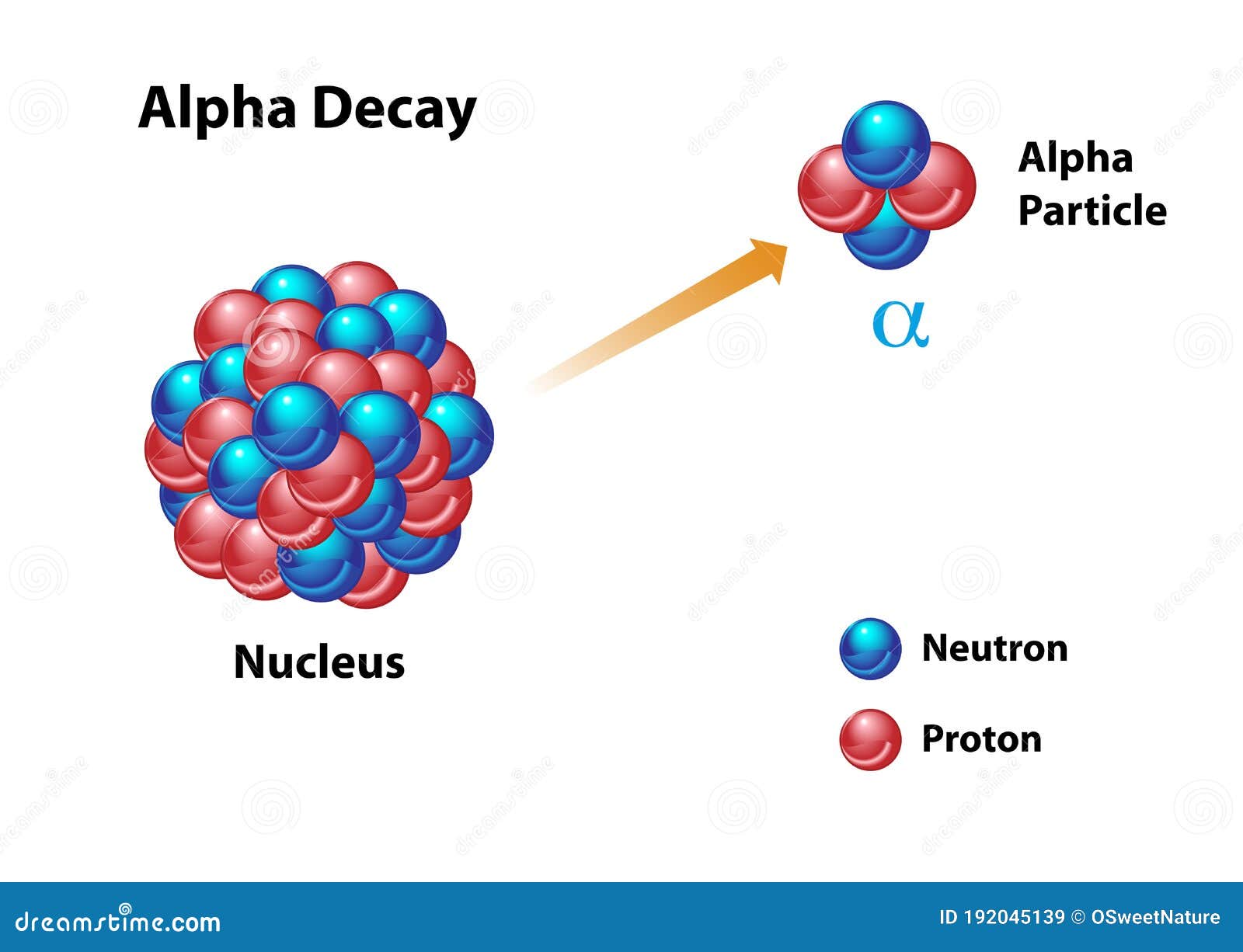
At their core, alpha particles are essentially helium nuclei, consisting of two protons and two neutrons tightly bound together. This structure grants them a distinct positive charge, twice the magnitude of a proton’s charge. The absence of electrons further contributes to their unique characteristics.
Origin and Emission: The Radioactive Decay Process
Alpha particles are born during a specific type of radioactive decay known as alpha decay. This process occurs within the nucleus of an unstable atom, where the strong nuclear force, responsible for holding protons and neutrons together, weakens. To regain stability, the nucleus expels an alpha particle, effectively transforming itself into a different element.
The Energetic Nature of Alpha Particles: High Kinetic Energy and Ionization Power
Alpha particles are emitted with substantial kinetic energy, a consequence of the strong forces within the nucleus. This high energy translates into a significant ability to ionize atoms, stripping them of their electrons as they traverse matter. This ionization power is a defining characteristic of alpha particles, contributing to their potential for causing biological damage.
Penetration and Range: The Limited Journey of Alpha Particles
Despite their high energy, alpha particles are relatively heavy and possess a limited range in matter. Their large size and positive charge lead to frequent collisions with atoms, causing them to lose energy rapidly. This limited penetration capability makes them effectively blocked by even thin layers of material, such as a sheet of paper or the outer layer of skin.
The Applications of Alpha Particles: Harnessing Their Power
The unique properties of alpha particles have led to their application in various fields:
- Radioactive Dating: Alpha decay provides a basis for radiometric dating, a technique used to determine the age of ancient artifacts, rocks, and fossils. By analyzing the ratio of parent isotopes to daughter isotopes, scientists can estimate the time elapsed since the decay process began.
- Smoke Detectors: Alpha particles are utilized in ionization-type smoke detectors. The particles ionize air molecules, creating a small current. When smoke particles enter the detector, they disrupt this current, triggering an alarm.
- Medical Applications: Alpha particles find applications in cancer therapy, specifically in targeted alpha therapy (TAT). This technique utilizes alpha-emitting isotopes attached to antibodies or other carriers, delivering a concentrated dose of radiation directly to cancerous cells.
The Risks Associated with Alpha Particles: Understanding the Potential for Harm
While alpha particles have valuable applications, their ionizing power also poses potential risks:
- Biological Damage: When alpha particles penetrate living tissue, their high ionization capacity can damage DNA molecules, potentially leading to mutations and cell death. The severity of damage depends on the energy of the alpha particle and the duration of exposure.
- Radiation Exposure: Exposure to alpha particles can lead to radiation sickness, a range of symptoms that can manifest as nausea, vomiting, fatigue, and even death in severe cases. The risks associated with alpha particle exposure are generally higher when the source is ingested or inhaled, allowing for greater interaction with internal tissues.
FAQs: Addressing Common Questions About Alpha Particles
Q: Are alpha particles dangerous?
A: Alpha particles can be dangerous if they enter the body, but they are relatively harmless if they remain outside. Their limited range in matter means they are easily stopped by a thin layer of material, such as skin or paper.
Q: How are alpha particles detected?
A: Alpha particles can be detected using various methods, including:
- Geiger counter: This instrument measures the ionization caused by alpha particles as they pass through a gas-filled tube.
- Scintillation detector: Alpha particles excite fluorescent materials, producing light pulses that can be detected.
- Cloud chamber: This device utilizes supersaturated vapor, where alpha particles leave visible tracks as they ionize the vapor molecules.
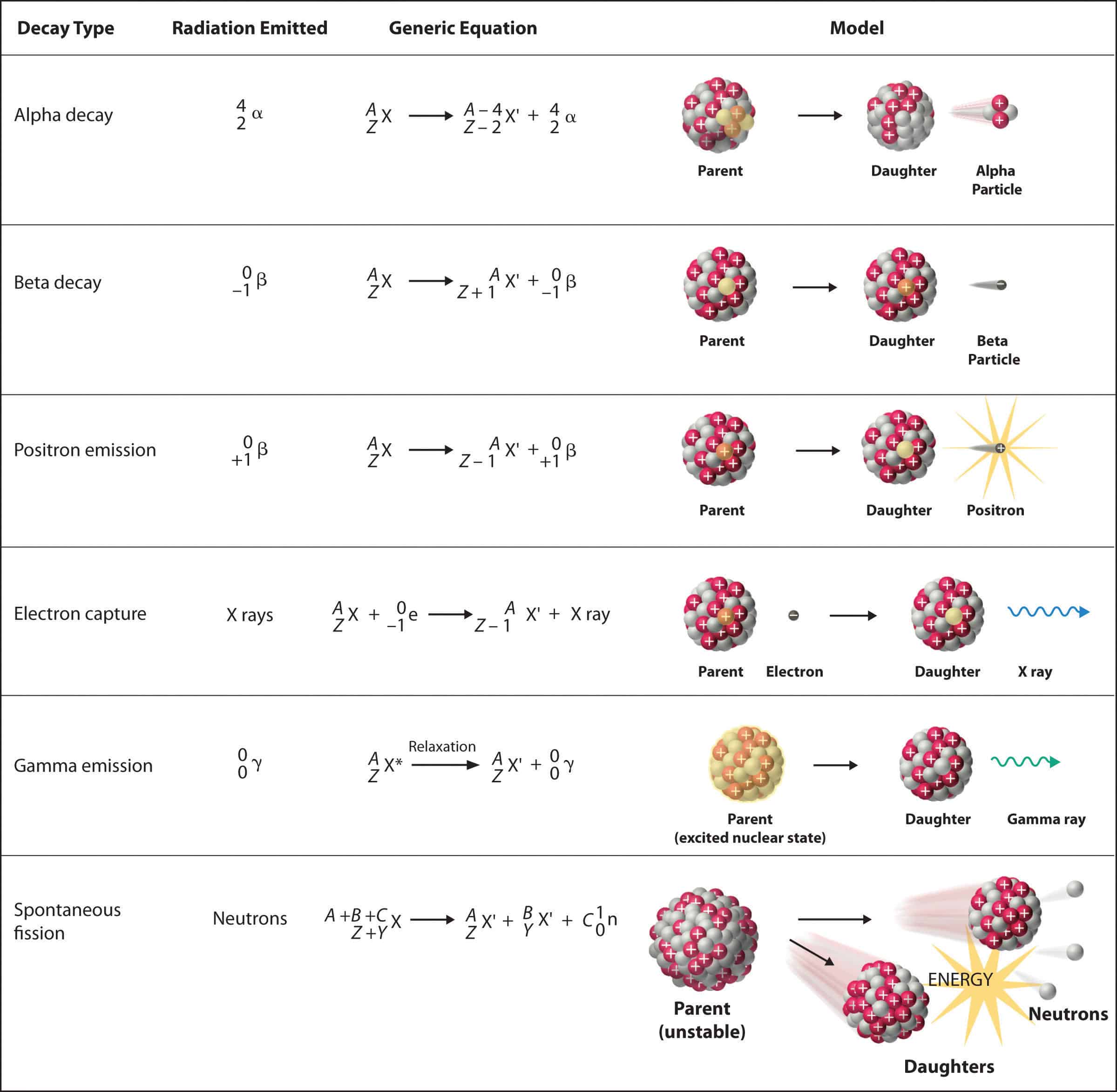
Q: What is the difference between alpha, beta, and gamma radiation?
A: Alpha, beta, and gamma radiation are all forms of radioactive decay, but they differ in their composition and properties:
- Alpha radiation: Consists of alpha particles, helium nuclei with two protons and two neutrons.
- Beta radiation: Consists of beta particles, which are either electrons or positrons (antimatter electrons).
- Gamma radiation: Consists of high-energy photons, electromagnetic radiation with no mass or charge.
Tips for Safe Handling of Alpha Sources:
- Minimize Exposure: Always handle alpha sources with appropriate protective equipment, such as gloves and lab coats.
- Distance: Maintain a safe distance from alpha sources to minimize exposure.
- Shielding: Use appropriate shielding materials, such as lead or concrete, to block alpha particles.
- Ventilation: Ensure adequate ventilation to minimize the risk of inhaling alpha particles.
Conclusion: Alpha Particles – A Powerful Force in the Nuclear Realm
Alpha particles, with their distinct structure and properties, play a vital role in the understanding of radioactive decay. Their high energy and ionizing power make them both a valuable tool in various fields and a potential source of harm. By understanding their nature and the risks associated with them, we can utilize their benefits while mitigating their potential dangers. As research continues to unravel the mysteries of the nuclear realm, alpha particles remain a fascinating subject of study, promising further insights into the fundamental processes that shape our world.





Closure
Thus, we hope this article has provided valuable insights into Unveiling the Essence of Alpha Particles: A Journey into the Heart of Radioactive Decay. We hope you find this article informative and beneficial. See you in our next article!