Unveiling The Essence Of Alpha Particles: A Journey Into The Heart Of Radioactive Decay
Unveiling the Essence of Alpha Particles: A Journey into the Heart of Radioactive Decay
Related Articles: Unveiling the Essence of Alpha Particles: A Journey into the Heart of Radioactive Decay
Introduction
With great pleasure, we will explore the intriguing topic related to Unveiling the Essence of Alpha Particles: A Journey into the Heart of Radioactive Decay. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Unveiling the Essence of Alpha Particles: A Journey into the Heart of Radioactive Decay
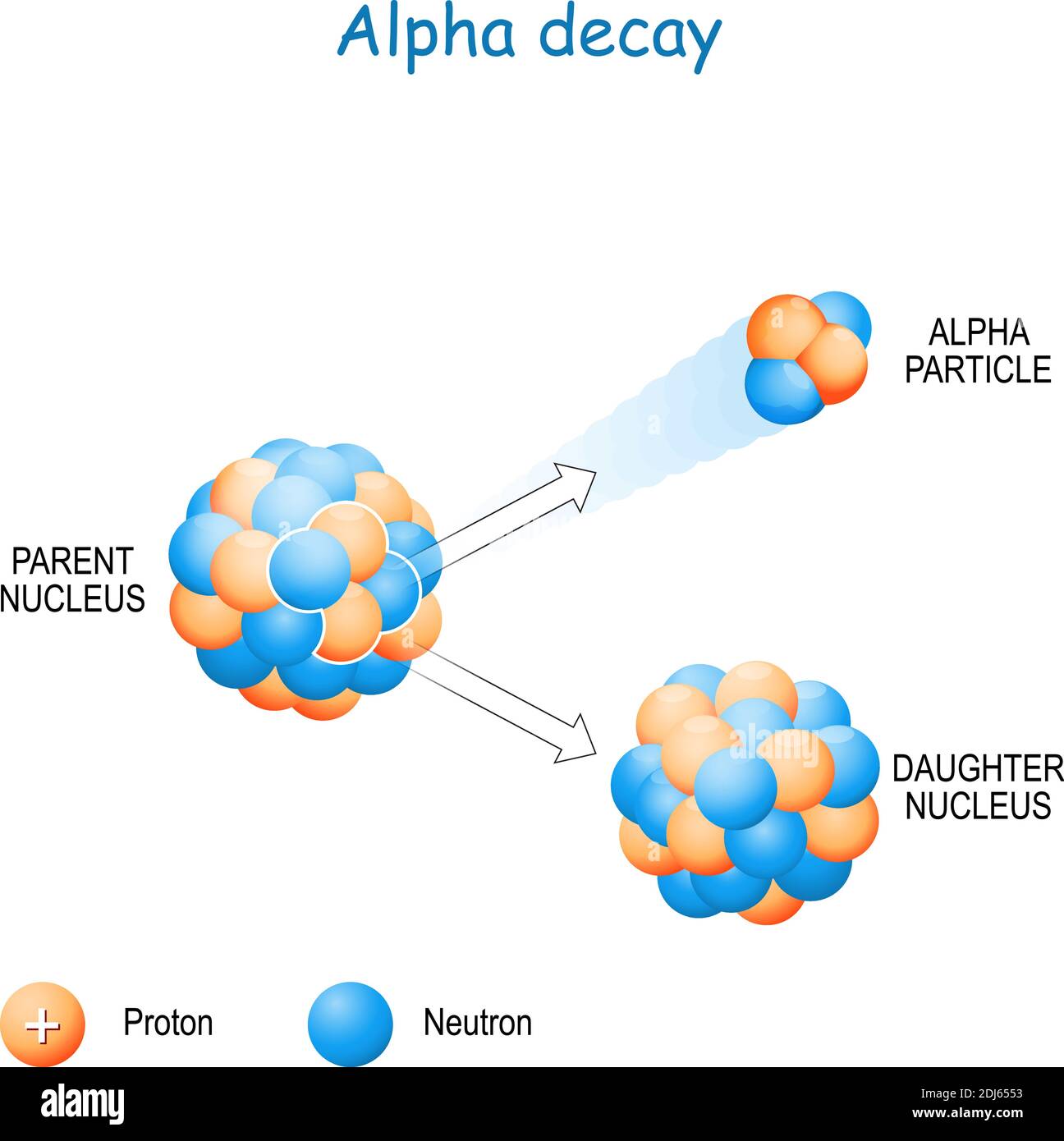
Alpha particles, a fundamental component of the radioactive decay process, play a crucial role in shaping the universe and influencing our understanding of nuclear physics. Their unique nature, consisting of two protons and two neutrons bound together, grants them distinct properties that have both scientific and practical implications. This exploration delves into the composition, behavior, and significance of alpha particles, illuminating their profound impact on various fields.
The Building Blocks of Alpha Particles: A Nucleus in Disguise
At the core of every atom lies the nucleus, a tightly packed assembly of protons and neutrons. These subatomic particles, collectively known as nucleons, are held together by the strong nuclear force, a powerful but short-range interaction that overcomes the electrostatic repulsion between protons. Alpha particles, in essence, are miniature nuclei, mirroring the structure of the helium atom’s nucleus. This intrinsic resemblance to a stable element’s nucleus is a key factor in understanding their behavior.
The Composition: A Helium Nucleus Unveiled
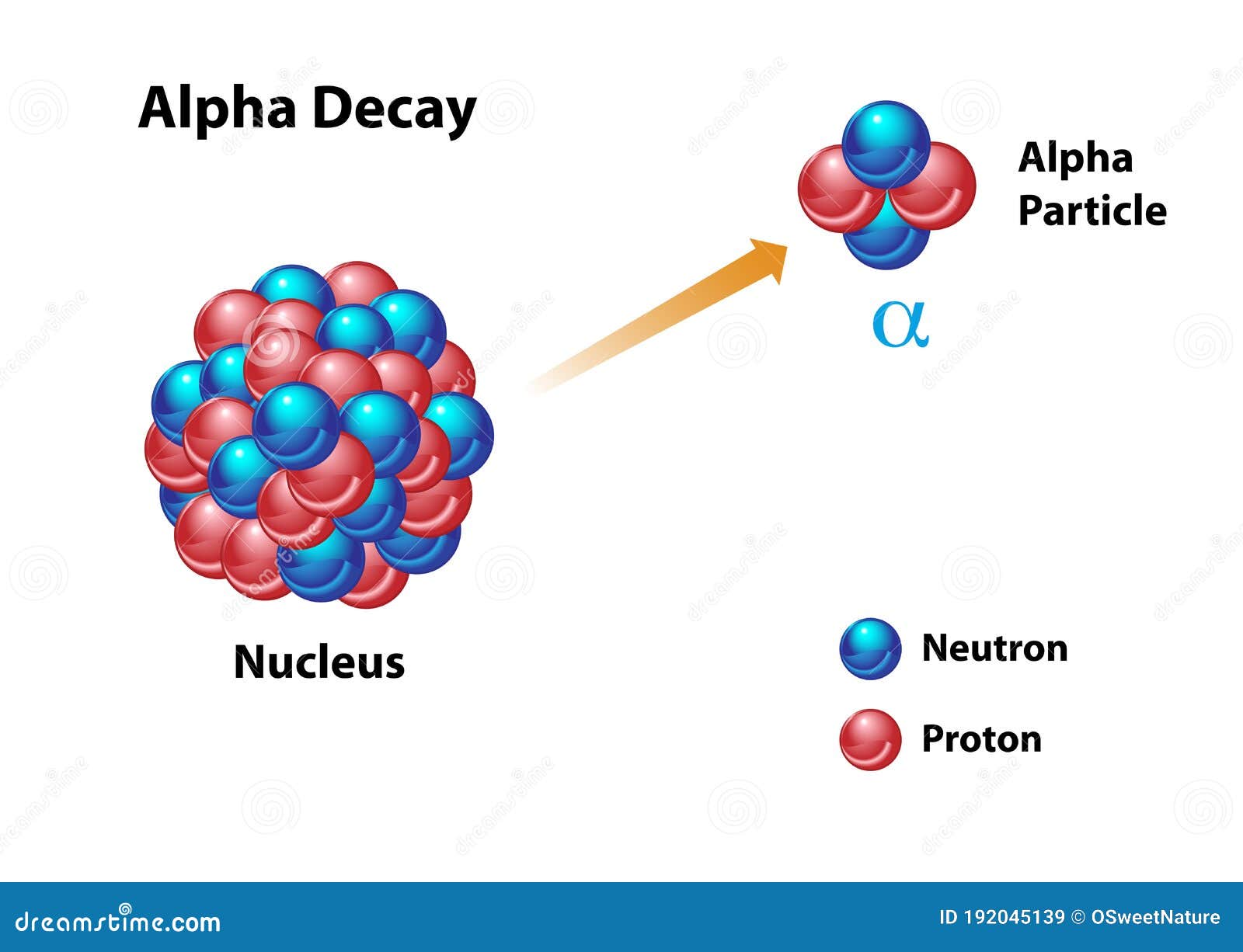
An alpha particle, symbolized as α, comprises two protons and two neutrons, identical to the nucleus of a helium-4 atom. This composition imparts a positive charge of +2e, where ‘e’ represents the elementary charge, and a mass of approximately 4 atomic mass units (amu). The strong nuclear force binds these nucleons together, creating a highly stable configuration that is relatively resistant to further decay.
The Origin: A Tale of Radioactive Decay
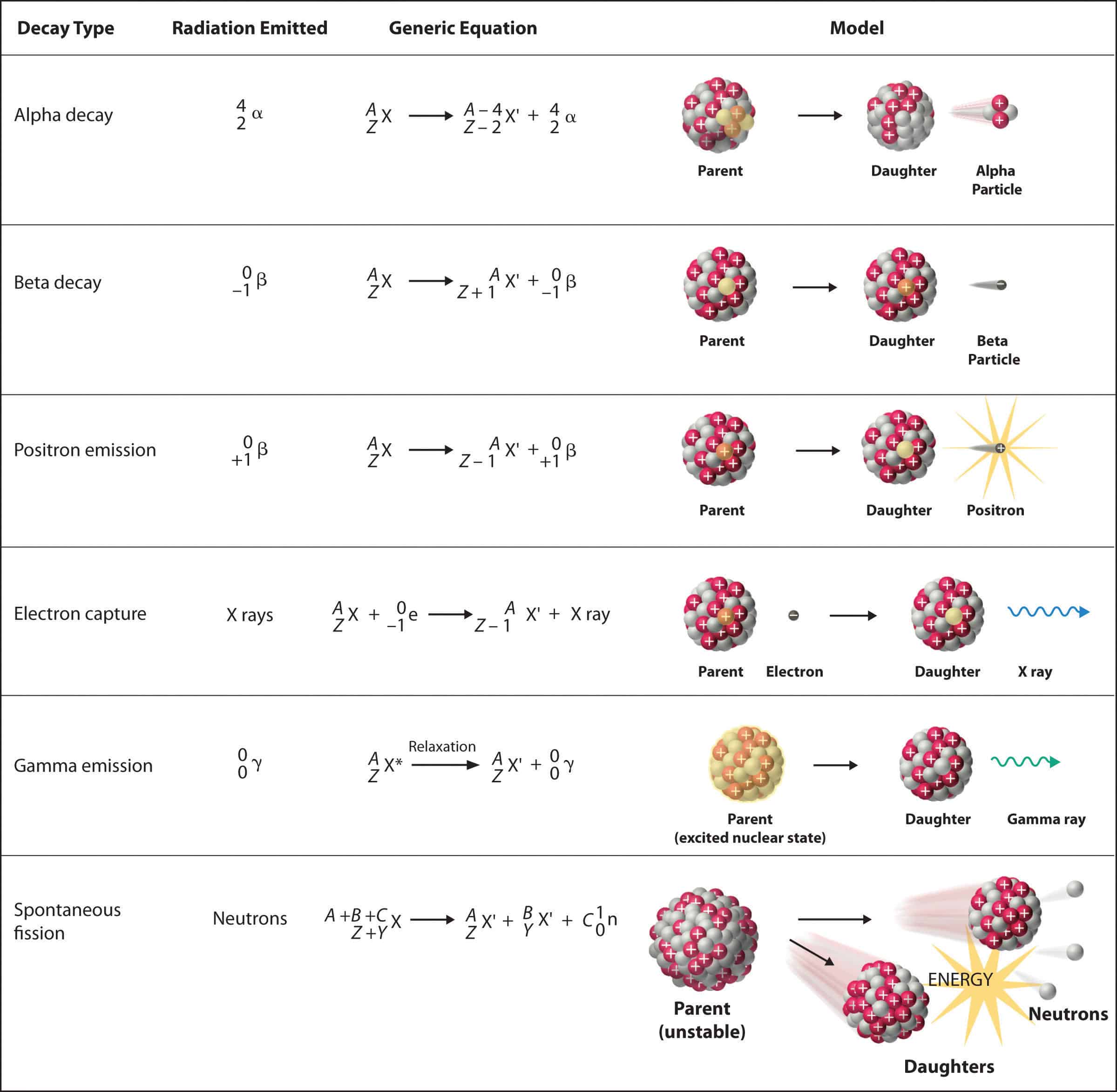
Alpha particles are born from the process of radioactive decay, a phenomenon where unstable atomic nuclei release energy and transform into different elements. This transformation can occur through various decay modes, with alpha decay being one of the most common. In alpha decay, an unstable nucleus ejects an alpha particle, essentially shedding its own nucleus, thereby transitioning into a different element with a lower atomic number. This process is driven by the instability of the parent nucleus, where the strong nuclear force is insufficient to overcome the electrostatic repulsion between protons.
The Journey: A High-Energy Projectile
Ejected from the parent nucleus with significant kinetic energy, alpha particles travel at high speeds, typically around 10% the speed of light. This energy is a consequence of the instability of the parent nucleus and the strong nuclear force’s influence. As the alpha particle traverses matter, it interacts with atoms, transferring its energy and causing ionization. This ionization process, where electrons are stripped from atoms, leads to the creation of ion pairs, marking the path of the alpha particle.
The Interaction: A Trail of Ionization
The high kinetic energy of alpha particles allows them to penetrate matter, but their relatively large size and charge limit their range. Due to their strong interaction with electrons, alpha particles ionize atoms along their path, leaving a trail of charged particles. This ionization process is responsible for the damaging effects of alpha radiation, as it can disrupt the delicate balance of chemical reactions within biological systems.
The Detection: A Tale of Scintillation
Alpha particles, due to their high ionization capacity, can be detected using various methods. One common technique involves the use of scintillation detectors. These detectors employ materials that emit light when struck by ionizing radiation, allowing for the visualization of the alpha particle’s path. This light emission, known as scintillation, is proportional to the energy deposited by the alpha particle, providing information about its origin and energy.
The Applications: From Smoke Detectors to Cancer Treatment
Alpha particles, despite their inherent danger, have found applications in various fields. Smoke detectors utilize the ionization properties of alpha particles to detect the presence of smoke. The alpha particles emitted by a small amount of americium-241 ionize the air within the detector. When smoke enters the chamber, it absorbs these ions, disrupting the current flow and triggering an alarm.
In medicine, alpha particle therapy is employed to treat certain types of cancer. Alpha particles, due to their high energy and short range, can effectively target and destroy cancerous cells with minimal damage to surrounding healthy tissues. This targeted approach minimizes side effects, making alpha particle therapy a promising treatment option for specific cancers.
The Risks: A Balancing Act Between Benefit and Danger
While alpha particles have valuable applications, their ionizing properties also pose significant risks. Exposure to alpha radiation can cause damage to biological tissues, leading to various health problems. The extent of damage depends on the dose and the duration of exposure. Alpha particles, due to their high ionization capacity, are particularly damaging to cells, as they can directly break DNA strands, leading to mutations and potentially cancer.
FAQs: Unveiling the Mysteries
Q: What is the difference between alpha particles and alpha radiation?
A: Alpha particles are the actual particles, consisting of two protons and two neutrons. Alpha radiation refers to the emission of these particles from a radioactive source.
Q: How can alpha particles be shielded?
A: Alpha particles, due to their relatively large size and charge, are easily shielded by thin layers of materials like paper or skin. Even a few centimeters of air can effectively stop them.
Q: Are alpha particles dangerous?
A: Alpha particles can be dangerous if they enter the body through inhalation, ingestion, or wounds. However, external exposure to alpha radiation is generally not a concern due to their limited range.
Q: How does alpha particle therapy work?
A: Alpha particle therapy uses the high energy and short range of alpha particles to target and destroy cancer cells. These particles are delivered directly to the tumor, minimizing damage to surrounding healthy tissues.
Tips: Navigating the World of Alpha Particles
1. Understanding the Source: Identifying the source of alpha radiation is crucial for assessing potential risks. Naturally occurring radioactive materials, like uranium and thorium, can emit alpha particles.
2. Recognizing the Signs: Alpha particles are invisible, but their effects can be detected through scintillation detectors or other specialized instruments.
3. Implementing Precautions: Wearing protective clothing and using appropriate shielding materials can minimize exposure to alpha radiation.
Conclusion: A Journey into the Heart of Nuclear Physics
Alpha particles, with their unique composition and behavior, hold a significant place in the world of nuclear physics. Their role in radioactive decay, their applications in various fields, and their potential risks highlight their multifaceted nature. Understanding alpha particles is crucial for advancing our knowledge of the atom, harnessing their potential, and mitigating their risks, ensuring a safer and more informed future.





Closure
Thus, we hope this article has provided valuable insights into Unveiling the Essence of Alpha Particles: A Journey into the Heart of Radioactive Decay. We appreciate your attention to our article. See you in our next article!