Unveiling The Wonders Of Chemistry: Experiments With Everyday Materials
Unveiling the Wonders of Chemistry: Experiments with Everyday Materials
Related Articles: Unveiling the Wonders of Chemistry: Experiments with Everyday Materials
Introduction
With great pleasure, we will explore the intriguing topic related to Unveiling the Wonders of Chemistry: Experiments with Everyday Materials. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Unveiling the Wonders of Chemistry: Experiments with Everyday Materials

Chemistry, the study of matter and its properties, is often perceived as a complex and abstract subject. However, the world around us is a vast chemical laboratory, teeming with opportunities to explore the fundamental principles of this fascinating science. Everyday household items, often taken for granted, can serve as powerful tools for engaging in hands-on experiments that bring chemistry to life.
This exploration delves into the realm of chemistry experiments utilizing common household materials, showcasing how these seemingly mundane objects can be transformed into catalysts for scientific discovery. The focus will be on elucidating the underlying chemical principles behind these experiments, emphasizing their educational value and potential for fostering a deeper understanding of the world around us.
The Allure of Household Chemistry Experiments:
The allure of conducting chemistry experiments with household items lies in their accessibility and practicality. They offer a unique opportunity to engage in scientific exploration without the need for specialized equipment or expensive reagents. This accessibility fosters a sense of wonder and curiosity, particularly in young minds, encouraging them to delve deeper into the world of science.
Furthermore, these experiments bridge the gap between theoretical concepts and real-world applications. By observing chemical reactions and phenomena in a familiar setting, individuals gain a concrete understanding of abstract scientific principles. The ability to relate these principles to everyday experiences enhances learning and promotes a deeper appreciation for the interconnectedness of science and everyday life.
Safety First: A Paramount Consideration:
While conducting chemistry experiments with household items can be a rewarding experience, safety should always be paramount. It is crucial to approach these experiments with caution and a thorough understanding of the potential risks involved.
- Adult Supervision: Experiments involving chemicals or heat should always be conducted under the supervision of a responsible adult.
- Proper Ventilation: Ensure adequate ventilation when working with volatile substances or chemicals that release fumes.
- Protective Gear: Wear appropriate safety gear such as gloves, goggles, and lab coats when handling chemicals or working with potentially hazardous materials.
- Clear Instructions: Follow instructions carefully and avoid deviations from the prescribed procedures.
- Proper Disposal: Dispose of chemicals and waste materials responsibly, following local regulations and guidelines.
A Glimpse into the World of Household Chemistry:
The following experiments illustrate how everyday materials can be utilized to explore fundamental chemical concepts:
1. The Magic of Baking Soda and Vinegar:
This classic experiment demonstrates the principles of acid-base reactions and the production of carbon dioxide gas.
- Materials: Baking soda (sodium bicarbonate), vinegar (acetic acid), a clear glass or container, a balloon.
-
Procedure:
- Fill the container with vinegar.
- Add a spoonful of baking soda to the balloon.
- Carefully stretch the balloon over the mouth of the container, ensuring that the baking soda does not fall into the vinegar.
- Gently lift the balloon, allowing the baking soda to fall into the vinegar.
- Observation: A vigorous reaction will occur, producing bubbles of carbon dioxide gas that inflate the balloon.
- Explanation: Baking soda is a base, while vinegar is an acid. When they react, they neutralize each other, releasing carbon dioxide gas. This gas is lighter than air, causing it to rise and inflate the balloon.
2. The Colorful World of Chromatography:
Chromatography, a technique used to separate mixtures based on their different affinities for a stationary phase, can be explored using household materials.
- Materials: Coffee filters, markers, water, a clear glass or jar.
-
Procedure:
- Draw lines across the bottom of the coffee filter using different colored markers.
- Carefully fold the filter into a cone shape and place it inside the glass or jar.
- Pour water into the glass or jar, ensuring that the bottom of the filter is submerged in the water.
- Observe the filter as the water travels up its surface.
- Observation: The different colored inks will separate into distinct bands, revealing the individual components of the mixture.
- Explanation: The water acts as the mobile phase, carrying the ink components up the filter paper. The different components of the ink have varying affinities for the filter paper (stationary phase), causing them to travel at different rates, resulting in their separation.
3. The Power of Density: The Egg Experiment:
This experiment demonstrates the concept of density and its influence on buoyancy.
- Materials: Eggs, a tall glass or jar, water, salt.
-
Procedure:
- Fill the glass or jar with water.
- Carefully place an egg in the water. Observe its behavior.
- Gradually add salt to the water, stirring until the egg floats.
- Observation: Initially, the egg sinks to the bottom of the glass. As salt is added, the density of the water increases, causing the egg to rise and eventually float.
- Explanation: The egg is denser than pure water, causing it to sink. When salt is added, it increases the density of the water. When the density of the water exceeds that of the egg, the egg floats.
4. The Crystallization of Salt:
Crystallization, the process of forming crystals from a solution, can be observed using common salt.
- Materials: Salt (sodium chloride), water, a small glass or container.
-
Procedure:
- Fill the glass or container with water.
- Gradually add salt to the water, stirring until no more salt dissolves.
- Place the container in a warm, sunny location.
- Observation: As the water evaporates, salt crystals will begin to form on the bottom and sides of the container.
- Explanation: When the water evaporates, the concentration of salt in the solution increases. Eventually, the solution becomes saturated, and the excess salt starts to crystallize out.
5. The Magic of Soap Bubbles:
Soap bubbles demonstrate the principles of surface tension and the properties of surfactants.
- Materials: Dish soap, water, a straw or pipe cleaner.
-
Procedure:
- Mix dish soap and water in a bowl.
- Dip the straw or pipe cleaner into the soapy mixture and gently blow air through it.
- Observation: Bubbles will form, exhibiting iridescent colors and a spherical shape.
- Explanation: Soap molecules have a unique structure, with one end attracted to water (hydrophilic) and the other end attracted to grease or oil (hydrophobic). When soap is added to water, it reduces the surface tension of the water, allowing bubbles to form.
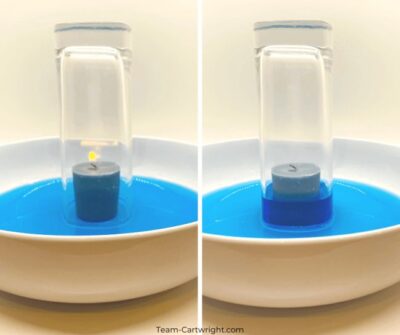
6. The Science Behind Candle Flames:
Candle flames provide an excellent opportunity to explore the principles of combustion and the chemical reactions involved.
- Materials: Candle, matches or a lighter, a glass jar or container.
-
Procedure:
- Light the candle.
- Carefully place the glass jar over the candle flame.
- Observation: The flame will gradually diminish and eventually extinguish.
- Explanation: The flame requires oxygen to burn. When the glass jar is placed over the candle, it cuts off the supply of oxygen, causing the flame to extinguish.
7. The Amazing Properties of Ice:
Ice, the solid form of water, exhibits unique properties that can be explored through simple experiments.
- Materials: Ice cubes, a glass or bowl, salt.
-
Procedure:
- Place an ice cube on a plate.
- Sprinkle salt on the ice cube.
- Observation: The salt will cause the ice to melt faster.
- Explanation: Salt lowers the freezing point of water. When salt is added to ice, it causes the ice to melt faster because the salt dissolves in the water, creating a solution with a lower freezing point.
8. The Chemical Reactions of Food Coloring:
Food coloring provides a colorful way to explore chemical reactions and the concept of diffusion.
- Materials: Food coloring, water, two clear glasses or containers.
-
Procedure:
- Fill one glass with water.
- Add a few drops of food coloring to the second glass.
- Carefully pour the colored water into the glass of plain water.
- Observation: The food coloring will gradually spread throughout the water, creating a gradient of color.
- Explanation: The food coloring molecules diffuse through the water, spreading out from the region of higher concentration to the region of lower concentration.
9. The Chemistry of Batteries:
Batteries utilize chemical reactions to produce electricity.
- Materials: A lemon or potato, copper wire, zinc-coated nail, a small LED light bulb.
-
Procedure:
- Insert the copper wire and the zinc-coated nail into the lemon or potato, ensuring they do not touch.
- Connect the ends of the wires to the terminals of the LED light bulb.
- Observation: The LED light bulb will light up.
- Explanation: The lemon or potato acts as an electrolyte, allowing the flow of ions between the copper and zinc electrodes. This flow of ions creates an electrical current that powers the LED light bulb.
10. The Magic of Slime:
Slime, a non-Newtonian fluid, exhibits interesting properties that can be explored through hands-on experimentation.
- Materials: White glue, borax solution (prepared by dissolving borax powder in water), a bowl, a spoon.
-
Procedure:
- Pour the white glue into the bowl.
- Gradually add the borax solution to the glue, stirring continuously.
- Observation: The mixture will gradually transform into a slime-like substance.
- Explanation: The borax solution reacts with the glue molecules, forming long chains that create the slime’s unique texture.
Benefits of Exploring Chemistry Through Everyday Experiments:
These experiments not only provide a hands-on learning experience but also offer numerous benefits, including:
- Developing Scientific Literacy: Conducting these experiments encourages critical thinking, observation, and data analysis skills, fostering a deeper understanding of scientific principles.
- Promoting Curiosity and Exploration: The accessibility of these experiments sparks curiosity and encourages individuals to ask questions, explore further, and delve into the world of science.
- Fostering Creativity and Innovation: These experiments provide opportunities to experiment, modify procedures, and explore alternative methods, promoting creativity and innovative thinking.
- Building Confidence and Problem-Solving Skills: The ability to conduct experiments and troubleshoot issues instills confidence and develops problem-solving skills.
- Making Science Relevant and Engaging: By demonstrating the relevance of chemistry in everyday life, these experiments make science more relatable and engaging, breaking down barriers to learning.
FAQs on Chemistry Experiments with Household Items:
1. What are the safety precautions to take when conducting chemistry experiments with household items?
Always prioritize safety when conducting chemistry experiments, especially those involving chemicals. Ensure adult supervision, proper ventilation, the use of appropriate protective gear, and careful adherence to instructions. Dispose of chemicals and waste materials responsibly.
2. Are all household items safe to use in chemistry experiments?
Not all household items are safe to use in experiments. It is crucial to research the properties of each material and its potential hazards before conducting an experiment. Avoid using potentially toxic or flammable substances.
3. How can I make these experiments more engaging for children?
Involve children in the planning and execution of the experiments, encouraging them to ask questions and explore their own ideas. Make the experiments interactive and visually appealing, using colorful materials and incorporating storytelling elements.
4. Can these experiments be used in educational settings?
Absolutely! These experiments can be valuable tools for teaching chemistry concepts in a hands-on and engaging manner. They provide opportunities for students to explore scientific principles, develop critical thinking skills, and connect theory to real-world applications.
5. How can I find more ideas for chemistry experiments with household items?
Numerous resources are available online and in libraries, including books, websites, and educational videos, providing a wealth of ideas for chemistry experiments using household items.
Tips for Conducting Successful Chemistry Experiments with Household Items:
- Plan Ahead: Before starting an experiment, gather all necessary materials and review instructions carefully.
- Start Simple: Begin with simple experiments and gradually progress to more complex ones as confidence and understanding grow.
- Record Observations: Keep a detailed record of observations, including changes in color, temperature, odor, and any other notable phenomena.
- Reflect on Results: After each experiment, reflect on the results, analyze the data, and draw conclusions based on the observations.
- Share Your Findings: Share your results with others, encouraging discussion and the sharing of ideas.
Conclusion:
Chemistry experiments with household items offer a unique and rewarding way to explore the wonders of science. By transforming everyday materials into tools for scientific discovery, these experiments foster a deeper understanding of fundamental chemical principles, promote curiosity and exploration, and make science more accessible and engaging. Embrace the potential of these experiments to ignite a passion for science and inspire a lifelong love of learning.







Closure
Thus, we hope this article has provided valuable insights into Unveiling the Wonders of Chemistry: Experiments with Everyday Materials. We appreciate your attention to our article. See you in our next article!