Acids in Our Homes: Unveiling the Chemistry of Everyday Objects
Related Articles: Acids in Our Homes: Unveiling the Chemistry of Everyday Objects
Introduction
In this auspicious occasion, we are delighted to delve into the intriguing topic related to Acids in Our Homes: Unveiling the Chemistry of Everyday Objects. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Acids in Our Homes: Unveiling the Chemistry of Everyday Objects

Acids are ubiquitous in our daily lives, playing a crucial role in various household tasks and processes. Often overlooked, these chemical compounds contribute to the functionality of numerous products and contribute significantly to maintaining cleanliness, hygiene, and even culinary delights. This article delves into the world of common household items that possess acidic properties, exploring their applications, benefits, and safety considerations.
Understanding Acids: A Foundation for Exploration
Before embarking on a journey through the acidic landscape of our homes, it is essential to understand the fundamental nature of acids. In chemistry, acids are substances that release hydrogen ions (H+) when dissolved in water. This release of hydrogen ions is what defines their acidic properties, resulting in a characteristic sour taste and the ability to react with bases to form salts and water.
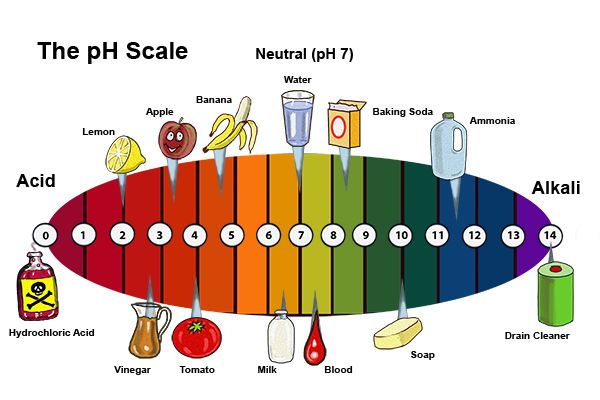
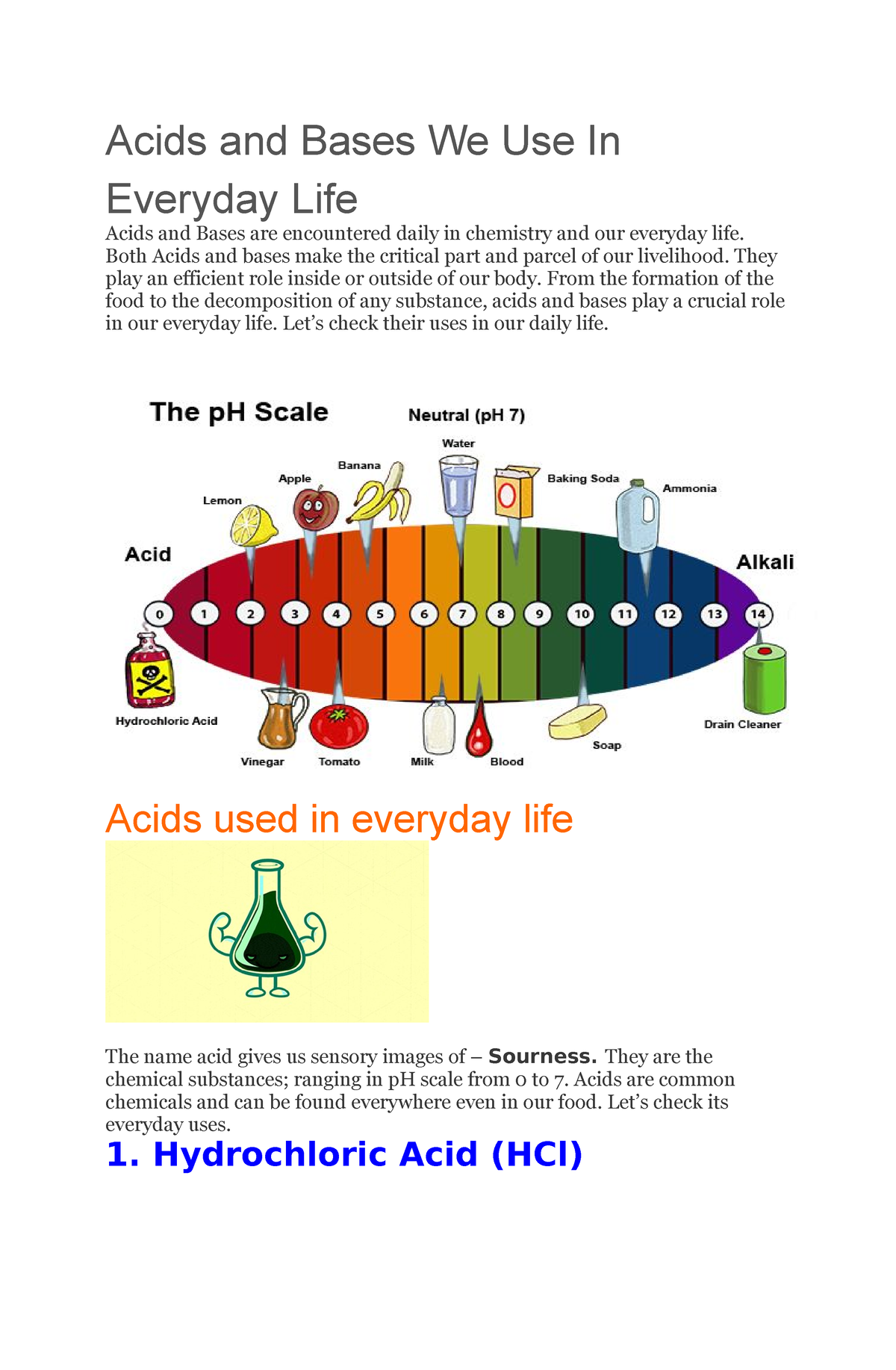
The strength of an acid is determined by its ability to donate hydrogen ions. Strong acids, such as hydrochloric acid (HCl), readily release hydrogen ions, while weak acids, like acetic acid (found in vinegar), release hydrogen ions less readily. The pH scale, ranging from 0 to 14, quantifies the acidity or alkalinity of a solution. A pH of 7 represents neutrality, while values below 7 indicate acidity, and values above 7 indicate alkalinity.
Common Household Items with Acidic Properties
Let’s now explore the fascinating world of acidic substances found within our homes, examining their roles and applications:
1. Vinegar: A Versatile Culinary and Cleaning Agent
Vinegar, a household staple, is a dilute solution of acetic acid, typically ranging from 4% to 5%. This acidic nature gives vinegar its characteristic pungent odor and sour taste, making it a popular ingredient in culinary preparations, particularly in salad dressings, marinades, and pickles.
Beyond its culinary applications, vinegar’s acidic properties make it an effective cleaning agent. Its ability to dissolve mineral deposits, such as calcium and magnesium, renders it suitable for cleaning hard water stains, removing grease, and deodorizing surfaces. Vinegar’s mild acidity also makes it a safe and effective disinfectant, particularly for surfaces that come into contact with food.
2. Citric Acid: A Natural Souring Agent and Cleaning Aid
Citric acid, a naturally occurring organic compound, is found in abundance in citrus fruits, particularly lemons and limes. This weak acid imparts the characteristic tart flavor to citrus fruits and is widely used as a natural souring agent in food and beverages.
Citric acid’s acidic properties also make it a valuable cleaning agent. It is effective in removing stains, brightening surfaces, and deodorizing. Its gentle nature makes it suitable for cleaning delicate surfaces, such as silverware and porcelain. Citric acid is also commonly used in cleaning products, such as bathroom cleaners and dishwashing detergents.
3. Lemon Juice: A Multifaceted Kitchen Essential
Lemon juice, a rich source of citric acid, is a versatile ingredient in both culinary and cleaning applications. Its acidic properties contribute to its refreshing flavor and its ability to brighten and enhance the taste of dishes.
Lemon juice is also a natural cleaning agent, capable of removing stains, deodorizing surfaces, and brightening metals. Its gentle acidity makes it suitable for cleaning delicate surfaces, such as countertops and silverware.
4. Carbonated Beverages: The Fizz of Acidity
Carbonated beverages, such as sodas and sparkling waters, contain carbonic acid, a weak acid that gives these drinks their characteristic fizz. Carbonic acid is formed when carbon dioxide gas dissolves in water, resulting in a slightly acidic solution.
While carbonated beverages are enjoyed for their refreshing taste and effervescence, their acidity can contribute to dental erosion if consumed excessively. The acidic nature of these drinks can also react with certain metals, potentially causing corrosion or discoloration.
5. Battery Acid: A Powerful Chemical with Cautionary Measures
Battery acid, typically sulfuric acid (H2SO4), is a highly corrosive strong acid with a wide range of applications, including the production of fertilizers, detergents, and explosives. Its corrosive nature necessitates extreme caution when handling battery acid.
Battery acid is commonly found in lead-acid batteries, which power vehicles and other equipment. It is essential to handle these batteries with care, as contact with battery acid can cause severe burns.
6. Stomach Acid: The Crucial Component of Digestion
Hydrochloric acid (HCl), a strong acid, is a vital component of gastric juice in the stomach. Its acidic nature helps to break down food, activate digestive enzymes, and kill harmful bacteria.
While stomach acid plays a crucial role in digestion, excessive production or insufficient protection of the stomach lining can lead to conditions like heartburn and ulcers.
7. Aspirin: Pain Relief with an Acidic Edge
Aspirin, a common over-the-counter pain reliever, is acetylsalicylic acid, a weak acid. Its acidic properties contribute to its analgesic and anti-inflammatory effects.
While aspirin is generally safe when used as directed, it can irritate the stomach lining, potentially causing heartburn or ulcers in some individuals.
8. Yogurt: A Probiotic Rich in Lactic Acid
Yogurt, a fermented dairy product, contains lactic acid, a weak acid produced by bacteria during the fermentation process. This acid gives yogurt its characteristic tangy flavor and contributes to its probiotic properties.
Lactic acid is also found in other fermented foods, such as sauerkraut and kimchi. Its presence in yogurt and other fermented foods contributes to their health benefits, including improved digestion and immune function.
9. Fruits: A Natural Source of Acids
Many fruits contain natural acids, contributing to their characteristic flavors and nutritional value. For example, oranges and grapefruits contain citric acid, while apples and pears contain malic acid. These acids play a role in the fruit’s growth and development, and they also contribute to their antioxidant properties.
10. Pickles: The Tang of Fermentation
Pickles, a popular condiment, are vegetables that have been fermented in a brine solution containing vinegar, salt, and other spices. The vinegar contributes to the pickle’s characteristic tangy flavor and acts as a preservative, inhibiting the growth of bacteria.
Safety Considerations and Responsible Use
While acids play a vital role in our daily lives, it is crucial to handle them with care and awareness.
- Skin and Eye Protection: Acids can cause irritation, burns, and damage to the skin and eyes. Always wear appropriate protective gear, such as gloves and goggles, when handling acidic substances.
- Dilution and Storage: Diluting strong acids with water can generate heat and potentially cause splashing. Always add acid to water, never water to acid, and store acids in appropriately labeled containers in well-ventilated areas.
- First Aid: In case of accidental contact with acids, immediately flush the affected area with copious amounts of water. Seek medical attention promptly.
FAQs: Common Household Acids
Q: Is it safe to use vinegar to clean delicate surfaces like marble or granite?
A: While vinegar is a generally safe cleaning agent, it can etch or dull the finish of some natural stone surfaces like marble or granite. It is recommended to test vinegar on an inconspicuous area before applying it to the entire surface.
Q: Can I use lemon juice to clean metal surfaces?
A: Lemon juice can be used to clean some metal surfaces, but its acidic nature can cause discoloration or damage to certain metals, such as copper or brass. It is recommended to test lemon juice on a small, inconspicuous area before applying it to the entire surface.
Q: Is it safe to drink carbonated beverages regularly?
A: While occasional consumption of carbonated beverages is generally safe, excessive consumption can contribute to dental erosion and other health problems. It is recommended to moderate intake and brush teeth regularly after consuming carbonated drinks.
Q: Can I use battery acid for cleaning purposes?
A: Battery acid is a highly corrosive substance and should never be used for cleaning purposes. It can cause severe burns and damage to surfaces.
Q: Can I use aspirin to treat a cut or wound?
A: Aspirin is not intended for topical use and should not be applied to cuts or wounds. Its acidic nature can irritate the skin and delay healing.
Q: Is it safe to eat yogurt every day?
A: Yogurt is a healthy and nutritious food that can be enjoyed daily. Its lactic acid content contributes to its probiotic properties, promoting gut health and digestion.
Tips for Using Acids Safely and Effectively
- Always read and follow product labels and instructions.
- Use appropriate protective gear, such as gloves and goggles, when handling acids.
- Dilute strong acids with water carefully, adding acid to water, never water to acid.
- Store acids in appropriately labeled containers in well-ventilated areas.
- Keep acids away from children and pets.
- In case of accidental contact with acids, immediately flush the affected area with copious amounts of water and seek medical attention promptly.
Conclusion: The Importance of Acids in Our Lives
Acids play a significant role in our daily lives, contributing to the functionality of numerous household items and processes. From the tangy flavor of vinegar to the cleaning power of citric acid, acids enhance our culinary experiences, maintain cleanliness, and even contribute to our health. By understanding the properties and safe handling of these chemical compounds, we can maximize their benefits while minimizing potential risks. Responsible use and awareness are key to ensuring the safe and effective utilization of acids in our homes.






Closure
Thus, we hope this article has provided valuable insights into Acids in Our Homes: Unveiling the Chemistry of Everyday Objects. We appreciate your attention to our article. See you in our next article!






























:max_bytes(150000):strip_icc()/rheumatoid-arthritis-diet-and-exercise-5094998_final-30997c03f3e94927898ceb065d0ee512.jpg)

















_Pounds_10/Yale_of_Beaufort_2023_04.09.2022_19.51_01.jpg)

























![Household Uses of Ammonia [Detailed Guide] Handy House Guides](https://handyhouseguides.com/wp-content/uploads/2022/10/Household-Uses-of-Ammonia-Detailed-Guide-feature-img.jpg)



